Cystic fibrosis
| Cystic fibrosis Classification and external resources | |
| ICD-10 | E84 |
|---|---|
| ICD-9 | 277 |
| OMIM | 219700 |
| DiseasesDB | 3347 |
| MedlinePlus | 000107 |
| eMedicine | ped/535 |
| MeSH | D003550 |
Cystic fibrosis (CF) is a multisystem hereditary disease that mainly affects the lungs and digestive system, causing progressive disability and for some, early death. Formerly known as cystic fibrosis of the pancreas, this entity has increasingly been labeled simply "cystic fibrosis." Average life expectancy is around 37 years, although improvements in treatments mean a baby born today with CF could live longer.[1] Median ages of survival for males is greater than 32 years and approximately 29 years for females.[2]
Difficulty breathing and insufficient enzyme secretion in the pancreas are the most common symptoms that patients present with. Thick mucus production as well as a less competent immune system result in frequent lung infections, which are treated, though not always cured, by oral and intravenous antibiotics and other medications. A multitude of other symptoms, including sinus infections, poor growth, diarrhea, and potential infertility (mostly in males, due to the condition congenital bilateral absence of the vas deferens) result from the effects of CF on other parts of the body. Often, symptoms of CF appear in infancy and childhood; these include meconium ileus, failure to thrive, and recurrent lung infections. However, 7 percent of patients in the United States are diagnosed during adulthood.[2]
Cystic fibrosis is one of the most common life-shortening, childhood-onset inherited diseases. In the United States, 1 in 3900 children is born with CF.[3] It's prevalence does vary with ethnicity. It is most common among Europeans and Ashkenazi Jews, being detected in 1 in every 3000 live births; it the most common genetic disease among such people. The disease is less common in African Americans (1 in every 17,000 live births) and even less common in the Asian population of Hawaii, where it presents in 1 in every 90,000 live births.[2]
Individuals with cystic fibrosis can be diagnosed prior to birth by genetic testing or in early childhood by a sweat chloride test. Newborn screening tests are increasingly common and effective. To date, there is no cure for CF, and most individuals with cystic fibrosis die young, many in their 20s and 30s, most prevalently from lung failure. Many new treatments are being introduced for easing the symptoms of CF and increasing the life expectancy of a person with the disease, which are discussed below.
The occurrence of cystic fibrosis reflects on how remarkably harmonious is the complex coordination in the human body normally. Cystic fibrosis occurs when there is a mutation in the CFTR gene, which is 180,000 base pairs long and creates a protein that is 1,480 amino acids long. The most common mutation (although there are over 1,400 that can produce CF) is ΔF508, which is a deletion of only three nucleotides that results in a loss of the single amino acid phenylalanine at the 508th position on the protein. ΔF508 creates a protein that does not fold normally and for a protein to function properly it must precisely fold into a particular three-dimensional shape. In the overwhelming preponderance of cases, it does so correctly.
The name cystic fibrosis refers to the characteristic "fibrosis" (tissue scarring) and cyst formation within the pancreas, first recognized in the 1930s.[4]
Basis of the disease
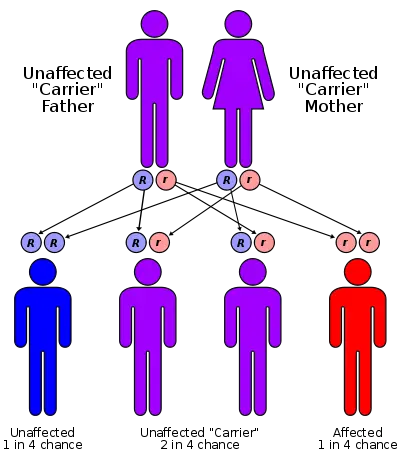
CF is mostly commonly (>70 percent) caused by a mutation in a gene located on chromosome 7, whose protein product is the cystic fibrosis transmembrane conductance regulator (CFTR). The mutation causes the deletion of three base pairs at position 508, resulting in the absence of the amino acid phenylalanine. Consequently, the cystic fibrosis transmembrane conductance regulator, the product of the CF gene, is defective. Although most people without CF have two working copies of the CFTR gene, only one is needed to prevent cystic fibrosis. CF develops when neither gene works normally. Therefore, CF is considered an autosomal recessive gene disease.
The disease can also be caused by over 1,400 other mutations in the CF gene; however, these mutations are relatively uncommon. (See pathophysiology section)
CFTR protein
The CFTR protein is an apical surface (faces lumen) chloride ion channel important in creating sweat, digestive juices, and mucus. It's role in different parts of the body are different, but regardless, it plays a crucial part in regulating the secretion or absorption of chloride ions into the lumen. The flow of chloride ions is important because it affects the osmotic flow of water.
In the lungs and airways, a normal CFTR channel secretes chloride ions into the lumen of the airways, resulting in the osmotic flow of water into the lumen as well. This results in the production of saline-like mucus, which is cleared from the airways by the cilia. In CF patients, the defective CFTR channel is unable to pump chloride ions out into the lumen. Consequently, water is not secreted either and mucus remains thick and viscous. The cilia lining the airways are unable to clear the thick mucus, thus causing a narrowing and clogging of the airways.
In the pancreas, the chloride ions are secreted into the pancreatic ducts for the exchange of a bicarbonate ion. This ion exchange, as it is referred to, controls the flow of water in and out of the cells. When defective channels are present in the pancreas, chloride ions are not secreted, blocking the flow of water out of the cells. The formation of thick mucus results, which clogs the duct and blocks the secretion of enzymes needed for digestion in the intestines. The same type of issues arise in the bile ducts and in the digestive tract itself.
The sweat glands are also greatly affected by CF. Affected patients present with salty sweat because of the lack of absorption of salt from the sweat by the cells. The CFTR channel, which normally absorbs salt from the sweat and is impermeable to water, is defective and unable to absorb salt. This results in salty tasting sweat.
Symptomatic diseases
The symptoms of cystic fibrosis depend on the age of an individual, the extent to which the disease affects specific organs, prior therapy, and the types of infections experienced. Cystic fibrosis affects the entire body and impacts breathing, digestion, and sexual reproduction. The newborn period may be marked by poor weight gain and intestinal blockage caused by thick feces. Other symptoms of CF appear during the remainder of childhood and early adulthood. These include continued problems with growth, the onset of lung disease, and increasing difficulties with absorption of vitamins and nutrients by the gastrointestinal tract, resulting in malabsorption and malnutrition. In addition, difficulties with fertility may become apparent when reproduction is attempted.
Lung and sinus disease
Cystic fibrosis patients suffer greatly from respiratory problems. Thick mucus, narrowed airways, wheezing during breathing, persistent cough, and infections are all common symptoms caused by the defective CFTR protein channel.
Lung disease usually results from clogging of airways due to thick mucus and inflammation caused by persistent bacterial infections. Inflammation and infection cause injury to the lungs and structural changes that lead to a variety of symptoms. In the early stages, incessant coughing, copious phlegm production, and decreased ability to exercise are common. Many of these symptoms occur when bacteria that normally inhabit the thick mucus grow out of control and cause pneumonia. Common bacteria cultured from CF patient that lower respiratory tract secretions are Haemophilus influenzae, S. aureus, and Pseudomonas aeruginosa.[5] In later stages of CF, changes in the architecture of the lung further exacerbate chronic difficulties in breathing.
Other symptoms include the coughing up blood (hemoptysis), changes in the major airways in the lungs (bronchiectasis), high blood pressure in the lung (pulmonary hypertension), heart failure, difficulties getting enough oxygen to the body, and respiratory failure requiring support with breathing masks such as bilevel positive airway pressure machines or mechanical ventilators.[5]
In addition to typical bacterial infections, people with CF more commonly develop other types of lung disease. Among these is allergic bronchopulmonary aspergillosis, in which the body's response to the common fungus Aspergillus fumigatus causes worsening of breathing problems. Another is infection with mycobacterium avium complex (MAC), a group of bacteria related to tuberculosis, which can cause further lung damage and does not respond to common antibiotics.
Aside from respiratory tract mucus, the mucus found in the paranasal sinuses is equally thick and may also cause blockage of the sinus passages, leading to infection. This often causes facial pain, fever, nasal drainage, and headaches. Individuals with CF may develop overgrowth of the nasal tissue (nasal polyps) due to inflammation from chronic sinus infections. These polyps can block the nasal passages and increase breathing difficulties.[6][7]
Gastrointestinal, liver, and pancreatic disease
Prior to prenatal and newborn screening, cystic fibrosis was often diagnosed when a newborn infant failed to pass feces (meconium). Meconium may completely block the intestines and cause serious illness. This condition, called meconium ileus, occurs in 10 percent of newborns with CF.[8] In addition, protrusion of internal rectal membranes (rectal prolapse) is more common in CF because of increased fecal volume, malnutrition, and increased intra–abdominal pressure due to coughing.[9]
The thick mucus seen in the lung has its counterpart in thickened secretions from the pancreas, an organ responsible for providing digestive juices that help break down food and prepare it for digestion by the small intestines. Thick mucus secretions of the pancreas block the movement of the digestive enzymes into the duodenum (first part of the small intestines) and result in irreversible damage to the pancreas, often with painful inflammation (pancreatitis).[10] The lack of digestive enzymes leads to difficulty absorbing nutrients with their subsequent excretion in the feces, a disorder known as malabsorption. Malabsorption leads to malnutrition and poor growth and development because of caloric loss. Individuals with CF also have difficulties absorbing the fat-soluble vitamins, such as vitamin A, vitamin D, vitamin E, and vitamin K. Stool fat content is high in CF patients due to the lack of fat absorption and consequent excretion.
In addition to the pancreatic problems, people with cystic fibrosis experience more heartburn, intestinal blockage, and constipation.[11] Older individuals with CF may also develop distal intestinal obstruction syndrome when thickened feces cause intestinal blockage.[12]
Thickened secretions also often cause liver problems. Bile, which is secreted by the liver to aid in digestion, may block the bile ducts, leading to liver damage due to the backing up of secretions. Over time, this can lead to cirrhosis, in which the liver fails to rid the blood of toxins and does not make important proteins, such as those responsible for bloodclotting.[13]
Endocrine disease and growth
The pancreas contains the islets of Langerhans, which are responsible for making insulin, a hormone that helps regulate blood glucose. Damage of the pancreas can lead to loss of the islet cells, leading to diabetes, which is unique to those with the disease.[14] Cystic Fibrosis Related Diabetes (CFRD), as it is known as, shares characteristics that can be found in Type 1 and Type 2 diabetics and is one of the principal non-pulmonary complications of CF.[15]
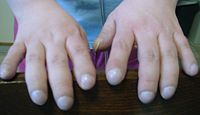
Vitamin D is involved in calcium and phosphorous regulation. Poor uptake of vitamin D from the diet because of malabsorption leads to the bone disease osteoporosis in which weakened bones are more susceptible to fractures.[16] In addition, people with CF often develop clubbing of their fingers and toes due to the effects of chronic illness and hypoxia (low oxygen) reaching their tissues.
Poor growth is a hallmark of CF. Children with CF typically do not gain weight or height at the same rate as their peers and occasionally are not diagnosed until investigation is initiated for this poor growth. The causes of growth failure are multi–factorial and include chronic lung infection, poor absorption of nutrients through the gastrointestinal tract, and increased metabolic demand due to chronic illness.
Infertility
Infertility affects both men and women. At least 97 percent of men with cystic fibrosis are infertile.[17] These men make normal sperm but are missing the tube (vas deferens) that connects the testes to the ejaculatory ducts of the penis.[18] Many men found to have congenital absence of the vas deferens during evaluation for infertility have a mild, previously undiagnosed form of CF.[19] Some women have fertility difficulties as well. These difficulties are attributed to thickened cervical mucus and/or malnutrition. In severe cases, malnutrition disrupts ovulation and causes amenorrhea.[20]
Diagnosis and monitoring
Cystic fibrosis may be diagnosed by newborn screening, sweat testing, or genetic testing. As of 2006 in the United States, ten percent of cases were diagnosed shortly after birth as part of newborn screening programs. The newborn screen identifies decreased amounts of the enzyme trypsin. However, most states and countries do not screen for CF routinely at birth. Therefore, most individuals are diagnosed after symptoms prompt an evaluation for cystic fibrosis. The most commonly used form of testing is the sweat test. Sweat testing involves application of a medication that stimulates sweating (pilocarpine) to one electrode of an apparatus and running electric current to a separate electrode on the skin. This process, called iontophoresis, causes sweating; the sweat is then collected on filter paper or in a capillary tube and analyzed for abnormal amounts of sodium and chloride. People with CF have increased amounts of sodium and chloride in their sweat. CF can also be diagnosed by identification of mutations in the CFTR gene.[21]
A multitude of tests is used to identify complications of CF and to monitor disease progression. X-rays and CAT scans are used to examine the lungs for signs of damage or infection. Sputum culture examination under a microscope is used to identify which bacteria are causing infection so that effective antibiotics can be given. Pulmonary function tests measure how well the lungs are functioning, and are used to measure the need for and response to antibiotic therapy. Blood tests can identify liver problems, vitamin deficiencies, and the onset of diabetes. Dual energy X-ray absorptiometry (DEXA scans) can screen for osteoporosis and testing for fecal elastase can help diagnose insufficient digestive enzymes.
Prenatal diagnosis
Couples who are pregnant or who are planning a pregnancy can themselves be tested for CFTR gene mutations to determine the likelihood that their child will be born with cystic fibrosis. Testing is typically performed first on one or both parents and, if the risk of CF is found to be high, testing on the fetus can then be performed. Cystic fibrosis testing is offered to many couples in the U.S.[22] The American College of Obstetricians and Gynecologists recommends testing for couples who have a personal or close family history of CF as well as couples at high risk because of their ethnicity.[23]
Because development of CF in the fetus requires each parent to pass on a mutated copy of the CFTR gene and because CF testing is expensive, testing is often performed on just one parent initially. If that parent is found to be a carrier of a CFTR gene mutation, the other parent is then tested to calculate the risk that their children will have CF. CF can result from more than a thousand different mutations and, as of 2006, it is not possible to test for each one. Testing analyzes the blood for the most common mutations such as ΔF508—most commercially available tests look for 32 or fewer different mutations. If a family has a known uncommon mutation, specific screening for that mutation can be performed. Because not all known mutations are found on current tests, a negative screen does not guarantee that a child will not have CF.[24] In addition, because the mutations tested are necessarily those most common in the highest risk groups, testing in lower risk ethnicities is less successful because the mutations commonly seen in these groups are less common in the general population.
Couples who are at high risk for having a child with CF will often opt to perform further testing before or during pregnancy. In vitro fertilization with pre-implantation genetic diagnosis offers the possibility to examine the embryo prior to its placement into the uterus. The test, performed 3 days after fertilization, looks for the presence of abnormal CF genes. If two mutated CFTR genes are identified, the embryo is excluded from embryo transfer and an embryo with at least one normal gene is implanted.
During pregnancy, testing can be performed on the placenta (chorionic villus sampling) or the fluid around the fetus (amniocentesis). However, chorionic villus sampling has a risk of fetal death of 1 in 100 and amniocentesis of 1 in 200,[25] so the benefits must be determined to outweigh these risks prior to going forward with testing. Alternatively, some couples choose to undergo third party reproduction with egg or sperm donors.
Pathophysiology
Cystic fibrosis occurs when there is a mutation in the CFTR gene. The protein created by this gene is anchored to the outer membrane (apical membrane) of cells in the sweat glands, lung, pancreas, and other affected organs. The protein spans this membrane and acts as a ion channel connecting the inner part of the cell (cytoplasm) to the surrounding fluid. This channel is primarily responsible for controlling the movement of chloride from inside to outside of the cell. When the CFTR protein does not work, chloride is trapped inside the cell in the lung and outside in the skin. Because chloride is negatively charged, positively charged ions also cannot cross into the cell because they are affected by the electrical attraction of the chloride ions. Sodium is the most common ion in the extracellular space and the combination of sodium and chloride creates the salt, which is lost in high amounts in the sweat of individuals with CF. This lost salt forms the basis for the sweat test.[5]
How this malfunction of cells in cystic fibrosis causes the clinical manifestations of CF is not well understood. One theory suggests that the lack of chloride exodus through the CFTR protein leads to the accumulation of more viscous, nutrient–rich mucus in the lungs, which allows bacteria to hide from the body's immune system. Another theory proposes that the CFTR protein failure leads to a paradoxical increase in sodium and chloride uptake, which, by leading to increased water re-absorption, creates dehydrated and thick mucus. Yet another theory focuses on abnormal chloride movement out of the cell, which also leads to dehydration of mucus, pancreatic secretions, biliary secretions, and so forth. These theories all support the observation that the majority of the damage in CF is due to blockage of the narrow passages of affected organs with thickened secretions. These blockages lead to remodeling and infection in the lung, damage by accumulated digestive enzymes in the pancreas, blockage of the intestines by thick feces, and so forth.[5]
The role of chronic infection in lung disease
The lungs of individuals with cystic fibrosis are colonized and infected by bacteria from an early age. These bacteria, which often spread among individuals with CF, thrive in the altered mucus, which collects in the small airways of the lungs. This mucus encourages the development of bacterial micro-environments (biofilms) that are difficult for immune cells (and antibiotics) to penetrate. The lungs respond to repeated damage by thick secretions and chronic infections by gradually remodeling the lower airways (bronchiectasis), making infection even more difficult to eradicate.[26]
Over time, both the types of bacteria and their individual characteristics change in individuals with CF. Initially, common bacteria such as Staphylococcus aureus and Hemophilus influenzae colonize and infect the lungs. Eventually, however, Pseudomonas aeruginosa (and sometimes Burkholderia cepacia) dominates. Once within the lungs, these bacteria adapt to the environment and develop antibiotic resistance to commonly used antibiotics. Pseudomonas can develop special characteristics that allows the formation of large colonies—these strains are known as "mucoid" Pseudomonas and are rarely seen in people who do not have CF.[27]
One way in which infection has spread is by passage between different individuals with CF.[28] In the past, people with CF often participated in summer "CF Camps" and other recreational gatherings.[29][30] A famous outbreak in the United Kingdom was reported in 1990-1992. A patient acquired the B. cepacia complex strain during summer camp in Canada and later spread it during weekly fitness classes in the UK.[31] Hospitals grouped patients with CF into common areas and routine equipment (such as nebulizers)[32] was not sterilized between individual patients.[33] This led to transmission of more dangerous strains of bacteria among groups of patients. As a result, individuals with CF are routinely isolated from one another in the health care setting and health care providers are encouraged to wear gowns and gloves when examining patients with CF in order to limit the spread of virulent bacterial strains.[34] Often, patients with particularly damaging bacteria will attend clinics on different days and in different buildings than those without these infections.
Molecular biology
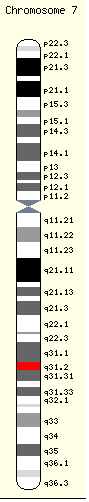
The CFTR gene is found at the q31.2 locus of chromosome 7, is 180,000 base pairs long, and creates a protein that is 1,480 amino acids long. The most common mutation, ΔF508, is a deletion (Δ) of three nucleotides that results in a loss of the amino acid phenylalanine (F) at the 508th (508) position on the protein. This mutation accounts for seventy percent of CF worldwide and 90 percent of cases in the United States. There are over 1,400 other mutations that can produce CF, however. In Caucasian populations, the frequency of mutations is as follows:[35] ! Mutation (Frequency worldwide)| ΔF508 (66.0%) | G542X (2.4%) | G551D (1.6%) | N1303K (1.3%) | W1282X (1.2%).
There are several mechanisms by which these mutations cause problems with the CFTR protein. ΔF508, for instance, creates a protein that does not fold normally and is degraded by the cell. Several mutations that are common in the Ashkenazi Jewish population result in proteins that are too short because translation (production) is ended prematurely. Less common mutations produce proteins that do not use energy normally, do not allow chloride to cross the membrane appropriately, or are degraded at a faster rate than normal. Mutations may also lead to fewer copies of the CFTR protein being produced.[5]
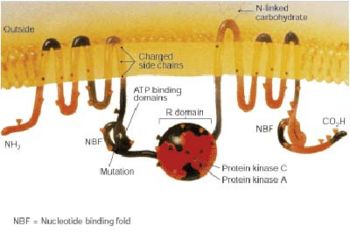
Structurally, CFTR is a type of gene known as an ATP-binding cassette transporter gene, or an ABC gene.[5] Its protein possesses two ATP-hydrolyzing domains which allows the protein to use energy in the form of adenosine triphosphate (ATP). It also contains two domains comprised of 6 alpha helices apiece which allow the protein to cross the cell membrane. A regulatory binding site on the protein allows activation by phosphorylation, mainly by cAMP-dependent protein kinase.[5] The C-terminal end (often referred to as the carboxyl terminal) of the protein is anchored to the cytoskeleton by a PDZ domain interaction.[36]
Treatment
The most consistent aspect of therapy in cystic fibrosis is limiting and treating the lung damage caused by thick mucus and infection with the goal of maintaining quality of life. Intravenous therapy, inhaled, and oral antibiotics are used to treat chronic and acute infections. Mechanical devices and inhalational medications are used to alter and clear the thickened mucus. Other aspects of CF therapy involve treatment of diabetes with insulin, pancreatic disease with enzyme replacement, and infertility with advanced reproductive techniques. In addition, therapies such as organ transplantation and gene therapy aim to cure some of the effects of cystic fibrosis.
Antibiotics to treat lung disease
Antibiotics are given whenever pneumonia is suspected or there has been a decline in lung function. Antibiotics are often chosen based on information about prior infections. Many bacteria common in cystic fibrosis are resistant to multiple antibiotics and require weeks of treatment with intravenous antibiotics such as vancomycin, tobramycin, meropenem, ciprofloxacin, and piperacillin. This prolonged therapy often necessitates hospitalization and insertion of a more permanent intravenous (IV) lines such as a peripherally inserted central catheter (PICC line) or Port-a-Cath. Inhaled therapy with antibiotics such as tobramycin and colistin is often given for months at a time in order to improve lung function by impeding the growth of colonized bacteria.[37][38] Oral antibiotics such as ciprofloxacin or azithromycin are sometimes given to help prevent infection or to control ongoing infection.[39] Some individuals spend years between hospitalizations for antibiotics, while others require several antibiotic treatments each year.
Several common antibiotics such as tobramycin and vancomycin can cause ototoxicity (hearing loss) or kidney problems with long-term use. In order to prevent these side effects, the amount of antibiotics in the blood are routinely measured and adjusted accordingly.
Other methods to treat lung disease
Several mechanical techniques are used to dislodge sputum and encourage its expectoration. In the hospital setting, physical therapy is utilized; a therapist pounds an individual's chest with his or her hands several times a day (chest percussion). Devices that recreate this percussive therapy include the ThAIRapy Vest and the intrapulmonary percussive ventilator (IPV). Newer methods such as Biphasic Cuirass Ventilation and associated clearance mode available in such devices, now integrate a cough assistance phase, as well as a vibration phase for dislodging secretions. Biphasic Cuirass Ventilation is also shown to provide a bridge to transplantation. These are portable and adapted for home use.[40] Aerobic exercise is of great benefit to people with cystic fibrosis. Not only does exercise increase sputum clearance, but it improves cardiovascular and overall health.
Aerosolized medications which help loosen secretions include dornase alfa and hypertonic saline.[41] Dornase is a recombinant human deoxyribonuclease that breaks down DNA in the sputum, thus decreasing its viscosity.[42] N-Acetylcysteine may also decrease sputum viscosity, but research and experience have shown its benefits to be minimal. Albuterol and ipratropium bromide are inhaled to increase the size of the small airways by relaxing the surrounding muscles.
Other inhalation treatments have shown promise for improved mucus clearance. Inhalation of hypertonic saline solution has produced increased mucus clearance and lung function in CF patients. The use of bronchodilators prior to inhalation can aid in therapy as well.[43]
As lung disease worsens, breathing support from machines may become necessary. Individuals with CF may need to wear special masks at night that help push air into their lungs. These machines, known as bilevel positive airway pressure (BiPAP) ventilators, help prevent low blood oxygen levels during sleep. BiPAP may also be used during physical therapy to improve sputum clearance.[44] During severe illness, people with CF may need to have a tube placed in their throats and their breathing supported by a ventilator.
Treatment of other aspects of CF
Newborns with meconium ileus typically require surgery, while adults with distal intestinal obstruction syndrome typically do not. Treatment of pancreatic insufficiency by replacement of missing digestive enzymes allows the duodenum to properly absorb nutrients and vitamins that would otherwise be lost in the feces. Even so, most individuals with CF take additional amounts of vitamins A, D, E, and K, and eat high calorie meals. It should be noted, however, that nutritional advice given to patients is, at best, mixed. Often, literature encourages the eating of high-fat foods without differentiating between saturated and unsaturated fats/ trans-fats: This lack of clear information runs counter to health advice given to the general population, and creates the risk of further serious health problems for people with cystic fibrosis as they grow older. So far, no large-scale research has been carried out into the incidence of atherosclerosis and coronary heart disease in adults with cystic fibrosis.
The diabetes mellitus common to many CF patients is typically treated with insulin injections or an insulin pump.[45] Development of osteoporosis can be prevented by increased intake of vitamin D and calcium and can be treated by bisphosphonates.[46] Poor growth may be avoided by insertion of a feeding tube for increasing calories through supplemental feeds or by administration of injected growth hormone.[47]
Sinus infections are treated by prolonged courses of antibiotics. The development of nasal polyps or other chronic changes within the nasal passages may severely limit airflow through the nose. Sinus surgery is often used to alleviate nasal obstruction and to limit further infections. Nasal steroids such as fluticasone are used to decrease nasal inflammation.[48] Female infertility may be overcome by in vitro fertilization technology, particularly embryo transfer techniques. Male infertility may be overcome with intracytoplasmic sperm injection.[49] Third party reproduction is also a possibility for women with CF.
Transplantation and gene therapy
Lung transplantation often becomes necessary for individuals with cystic fibrosis as lung function and exercise tolerance declines. Although single lung transplantation is possible in other diseases, individuals with CF must have both lungs replaced because the remaining lung would contain bacteria that could infect the transplanted lung. A pancreatic or liver transplant may be performed at the same time in order to alleviate liver disease and/or diabetes.[50] Lung transplantation is considered when lung function approaches a point where it threatens survival or requires assistance from mechanical devices.[51]
Gene therapy holds promise as a potential avenue to cure cystic fibrosis. Gene therapy attempts to place a normal copy of the CFTR gene into affected cells. Studies have shown that to prevent the lung manifestations of cystic fibrosis, only 5–10% the normal amount of CFTR gene expression is needed.[52] Many approaches have been theorized and several clinical trials have been initiated but, as of 2006, many hurdles still exist before gene therapy can be successful.[53]
Epidemiology
Cystic fibrosis is the most common life-limiting autosomal recessive disease among people of European heritage. In the United States, approximately 30,000 individuals have CF; most are diagnosed by six months of age. Canada has approximately 3,000 citizens with CF. As mentioned earlier, the occurrence of the disease varies with ethnicity. Approximately 1 in 25 people of European descent and 1 in 29 people of Ashkenazi Jewish descent is a carrier of a cystic fibrosis mutation. Although CF is less common in these groups, approximately 1 in 46 Hispanics, 1 in 65 Africans, and 1 in 90 Asians carry at least one abnormal CFTR gene.[54][55][56]
Cystic fibrosis is diagnosed in males and females equally. For unclear reasons, males tend to have a longer life expectancy than females.[57] Life expectancy for people with CF depends largely upon access to health care. In 1959, the median age of survival of children with cystic fibrosis was six months. In the United States, the life expectancy for infants born in 2006 with CF is 36.8 years, based upon data compiled by the Cystic Fibrosis Foundation.[58] In developed countries, people with CF live to a similar age. However, the life expectancy in underdeveloped countries is much less—the majority of individuals with CF do not live past the age of 10.
The Cystic Fibrosis Foundation also compiles lifestyle information about American adults with CF. In 2004, the foundation reported that 91 percent had graduated high school and 54 percent had at least some college education. Employment data revealed 12.6 percent of adults were disabled and 9.9 percent were unemployed. Marital information showed that 59 percent of adults were single and 36 percent were married or living with a partner. In 2004, 191 American women with CF were pregnant.
Theories about the prevalence of CF
The ΔF508 mutation is estimated to be up to 52,000 years old.[59] Numerous hypotheses have been advanced as to why such a lethal mutation has persisted and spread in the human population. Other common autosomal recessive diseases such as sickle cell anemia have been found to protect carriers from other diseases, a concept known as heterozygote advantage. Resistance to the following have all been proposed as possible sources of heterozygote advantage:
- Cholera: With the discovery that cholera toxin requires normal host CFTR proteins to function properly, it was hypothesized that carriers of mutant CFTR genes benefited from resistance to cholera and other causes of diarrhea.[60] Further studies have not confirmed this hypothesis.[61][62]
- Typhoid: Normal CFTR proteins are also essential for the entry of Salmonella typhi into cells,[63] suggesting that carriers of mutant CFTR genes might be resistant to typhoid fever. No in vivo study has yet confirmed this. In both cases, the low level of cystic fibrosis outside of Europe, in places where both cholera and typhoid fever are endemic, is not immediately explicable.
- Diarrhea: It has also been hypothesized that the prevalence of CF in Europe might be connected with the development of cattle domestication. In this hypothesis, carriers of a single mutant CFTR chromosome had some protection from diarrhea caused by lactose intolerance, prior to the appearance of the mutations that created lactose tolerance.[64]
- Tuberculosis: Poolman and Galvani from Yale University have added another possible explanation—that carriers of the gene have some resistance to TB.[65][66]
History
Although the entire clinical spectrum of CF was not recognized until the 1930s, certain aspects of CF were identified much earlier. Indeed, literature from Germany and Switzerland in the 1700s warned "Wehe dem Kind, das beim Kuß auf die Stirn salzig schmekt, er ist verhext und muss bald sterben," which translates to "Woe is the child kissed on the brow who tastes salty, for he is cursed and soon must die," recognizing the association between the salt loss in CF and illness. Carl von Rokitansky described a case of fetal death with meconium peritonitis, complication of meconium ileus associated with cystic fibrosis. Meconium ileus was first described in 1905 by Karl Landsteiner.[67] In 1936, Guido Fanconi published a paper describing a connection between celiac disease, cystic fibrosis of the pancreas, and bronchiectasis.[68]
In 1938, Dorothy Hansine Andersen published an article titled, "Cystic fibrosis of the pancreas and its relation to celiac disease: A clinical and pathological study" in the American Journal of Diseases of Children. In her paper, she described the characteristic cystic fibrosis of the pancreas correlated it with the lung and intestinal disease prominent in CF.[69] She also first hypothesized that CF was a recessive disease and first used pancreatic enzyme replacement to treat affected children. In 1952, Paul di Sant' Agnese discovered abnormalities in sweat electrolytes; the sweat test was developed and improved over the next decade.[70]
In 1988, the first mutation for CF, ΔF508, was discovered by Francis Collins, Lap-Chee Tsui, and John R. Riordan on the seventh chromosome. Research has subsequently found over 1000 different mutations that cause CF. Lap-Chee Tsui led a team of researchers at the Hospital for Sick Children in Toronto that discovered the gene responsible for CF in 1989. Cystic fibrosis represents the first genetic disorder elucidated strictly by the process of reverse genetics. Because mutations in the CFTR gene are typically small, classical genetics techniques were not able to accurately pinpoint the mutated gene.[71] Using protein markers, gene linkage studies were able to map the mutation to chromosome 7. Chromosome walking and chromosome jumping techniques were then used to identify and sequence the gene.[72]
CF is known in the United States as 65 Roses, a copyrighted phrase popularized by the Cystic Fibrosis Foundation. The Foundations says the phrase came into being when it was used by a young boy who had overheard his mother speaking of his illness. He later informed her that he knew she was working to help with "sixty-five roses."[73] The term has since been used as a symbol by organizations and families of cystic fibrosis victims.
Public awareness
The fight against cystic fibrosis has been a news story in France, where on April 30, 2007, the rising pop singer Grégory Lemarchal died from the illness at the age of 23. Grégory won the fourth round of Star Academy (equivalent of American Idol) in 2004, with a voting score of 80 percent at the grand final—a percentage unmatched in the history of the show (the runner-up, Lucie Silvas, only received 20 percent of votes). On May 4, a special television programme was broadcast on TF1 to commemorate his life, and its 10.5 million viewers were asked to donate money to help progress research into finding a cure. More than 7.5 million euros have been raised.[74] Following his death, his family started Association Grégory Lemarchal, an advocacy organization supporting people with cystic fibrosis.
Other organizations and support groups exist to raise public awareness about CF. The Cystic Fibrosis Foundation is one such organization. It aims to help patients and their families deal with the disease and to improve the quality of life of CF patients.
See also
Notes
- ↑ Cystic Fibrosis Trust, What is cystic fibrosis? The Cystic Fibrosis Trust (2007). Retrieved November 7, 2007.
- ↑ 2.0 2.1 2.2 Dennis L. Kasper et al., Harrison's Principles of Internal Medicine, 16th edition (McGraw-Hill Companies, 2007).
- ↑ Cystic Fibrosis Research, About cystic fibrosis, Cystic Fibrosis Research, Inc. (2007).
- ↑ D. Andersen, "Cystic fibrosis of the pancreas and its relation to celiac disease: A clinical and pathological study," American Journal of Diseases of Children 56 (1938): 344–399.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 S. M. Rowe, S. Miller, and E. J. Sorscher, "Cystic fibrosis," New England Journal of Medicine 352(19)(May 12 2005): 1992–2001. PMID 15888700
- ↑ M. Maldonado, A. Martinez, I. Alobid, and J. Mullol, "The Antrochoanal Polyp," Rhinology 42(4)(Dec. 2004): 178-182. Review. PMID 15626248.
- ↑ B. Ramsey and M. A. Richardson, "Impact of sinusitis in cystic fibrosis," Allergy Clin Immunol 90(3 Pt 2)(Sep. 1992): 547-552. PMID 1527348.
- ↑ E. Eggermont and K. De Boeck, "Small-intestinal abnormalities in cystic fibrosis patients," European Journal of Pediatrics 150 (12) (Oct. 1991): 824-828. Review. PMID 1743211.
- ↑ L. L. Kulczycki, and H. Shwachman, "Studies in cystic fibrosis of the pancreas: Occurrence of rectal prolapse," The New England Journal of Medicine 259(9)(Aug. 28 1958): 409-412. PMID 13578072.
- ↑ J. Cohn, K. Friedman, P. Noone, M. Knowles, L. Silverman, and P. Jowell, "Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis," The New England Journal of Medicine 339(10)(Sept. 3 1998): 653-658. PMID 9725922.
- ↑ A. Malfroot and I. Dab, "New insights on gastro-oesophageal reflux in cystic fibrosis by longitudinal follow up," Archives of Disease in Childhood 66(11)(Nov. 1991): 1339–45. PMID 175564.
- ↑ V. Khoshoo, and J. Udall Jr., "Meconium ileus equivalent in children and adults," The American Journal of Gastroenterology 89(2)(Feb. 1994): 153-157. PMID 8304294.
- ↑ S. Williams, D. Westaby, M. Tanner, and A. Mowat, "Liver and biliary problems in cystic fibrosis," British Medical Bulletin 48(4)(Oct. 1992): 877-892. PMID 1458306.
- ↑ A. Moran, K. Pyzdrowski, J. Weinreb, B. Kahn, S. Smith, K. Adams, and E. Seaquist, "Insulin sensitivity in cystic fibrosis," Diabetes 43(8)(Aug. 1994): 1020–1026. PMID 8039595.
- ↑ A. C. de Alves, R. Aguiar, A. Alves, and M. Santana, "Diabetes mellitus in patients with cystic fibrosis," Jornal Brasileiro de Pneumologia 33(2)(Apr. 2007): 213-221. PMID 17724542.
- ↑ C. Haworth, P. Selby, A. Webb, M. Dodd, H. Musson, R. McL Niven, G. Economou, A. Horrocks, A. Freemont, E. Mawer, and J. Adams, "Low bone mineral density in adults with cystic fibrosis," Thorax 54(11)(Nov. 1999): 961-7. PMID 10525552.
- ↑ T. McCallum, J. Milunsky, D. Cunningham, D. Harris, T. Maher, and R. Oates, "Fertility in men with cystic fibrosis: An update on current surgical practices and outcomes," Chest 118(4)(Oct. 2000): 1059–1062. PMID 11035677.
- ↑ J. Dodge, "Male fertility in cystic fibrosis," Lancet 346(8975)(Sep. 2, 1995): 587-588. PMID 7650999.
- ↑ A. Augarten, Y. Yahav, B. Kerem, D. Halle, J. Laufer, A. Szeinberg, J. Dor, S. Mashiach, E. Gazit, I. Madgar, "Congenital bilateral absence of vas deferens in the absence of cystic fibrosis," Lancet 344(1994): 1473–1474. PMID 7968122.
- ↑ M. Gilljam, M. Antoniou, J. Shin, A. Dupuis, M. Corey, and D. Tullis, "Pregnancy in cystic fibrosis. Fetal and maternal outcome," Chest 118(1)(Jul. 2000): 85–91. PMID 10893364.
- ↑ R. Stern, "The diagnosis of cystic fibrosis," The New England Journal of Medicine 336(1997): 487. PMID 9017943.
- ↑ Committee on Genetics, American College of Obstetricians and Gynecologists, ACOG Committee Opinion. Number 325. Update on carrier screening for cystic fibrosis," Obstetrics and Gynecology 106(December 2005): 1465. Retrieved November 7, 2007.
- ↑ American College of Obstetricians and Gynecologists and American College of Medical Genetics, Preconception and Prenatal Carrier Screening for Cystic Fibrosis. Clinical and Laboratory Guidelines (Washington, DC: American College of Obstetricians and Gynecologists, October 2001, ISBN 0915473747).
- ↑ S. Elias, G. Annas, and J. Simpson, "Carrier screening for cystic fibrosis: Implications for obstetric and gynecologic practice," American Journal of Obstetrics and Gynecology 164(1994): 1077. PMID 2014829.
- ↑ A. Tabor, J. Philip, M. Madsen, J. Bang, E. Obel, and B. Norgaard-Pedersen, "Randomised controlled trial of genetic amniocentesis in 4606 low-risk women," Lancet 1(8493)(Jun. 7 1986): 1287–1293. PMID 2423826.
- ↑ L. Saiman, "Microbiology of early CF lung disease," Pediatric Respiratory Reviews 5 Suppl A(2004): S367-369. PMID 14980298.
- ↑ L. Saiman, "Microbiology of early CF lung disease," Pediatric Respiratory Reviews 5 Suppl A(2004): S367-369. PMID 14980298.
- ↑ B. Tummler, U. Koopmann, D. Grothues, H. Weissbrodt, G. Steinkamp, and H. von der Hardt, "Nosocomial acquisition of Pseudomonas aeruginosa by cystic fibrosis patients," Journal of Clinical Microbiology 29(6)(Jun 1991): 1265–1267. PMID 1907611.
- ↑ Centers for Disease Control and Prevention (CDC). "Pseudomonas cepacia at summer camps for persons with cystic fibrosis," MMWR Morbidity and Mortality Weekly Report 42(23)(Jun. 18 1993): 456-459. PMID 7684813.
- ↑ D. Pegues, L. Carson, O. Tablan, S. FitzSimmons, S. Roman, J. Miller, and W. Jarvis, "Acquisition of Pseudomonas cepacia at summer camps for patients with cystic fibrosis. Summer Camp Study Group," The Journal of Pediatrics 124(5 Pt 1)(May 1994): 694–702. PMID 7513755.
- ↑ C. Moser, and N. Hoiby, Prevention of cross-infection in cystic fibrosis: How relatively simple initiatives have improved the prognosis for people with CF in Denmark. Cystic Fibrosis Worldwide.
- ↑ C. Pankhurst, and J. Philpott-Howard, "The environmental risk factors associated with medical and dental equipment in the transmission of Burkholderia (Pseudomonas) cepacia in cystic fibrosis patients," The Journal of Hospital Infection 32(4)(Apr. 1996): 249-255. PMID 8744509.
- ↑ A. Jones, J. Govan, C. Doherty, M. Dodd, B. Isalska, T. Stanbridge, and A. Webb, "Identification of airborne dissemination of epidemic multi-resistant strains of Pseudomonas aeruginosa at a CF centre during a cross infection outbreak," Thorax 58(6)(Jun. 2003): 525-527. PMID 12775867.
- ↑ N. Hoiby, "Isolation and treatment of cystic fibrosis patients with lung infections caused by Pseudomonas (Burkholderia) cepacia and multi-resistant Pseudomonas aeruginosa," Netherlands Journal of Medicine 46(6)(Jun. 1995): 280-7. PMID 7643943.
- ↑ F.G. Araújo, F. C. Novaes, N. P. Santos, V. C. Martins, S. M. Souza, S. E. Santos, and A. K. Ribeiro-dos-Santos, "Prevalence of ΔF508, G551D, G542X, R553X mutations among cystic fibrosis patients in the North of Brazil," Brazilian Journal of Medical and Biological Research 38(2005): 11–15. PMID 15665983. Retrieved November 24, 2007.
- ↑ D. Short, K. Trotter, D. Reczek, S. Kreda, A. Bretscher, R. Boucher, M. Stutts, and S. Milgram, "An apical PDZ protein anchors the cystic fibrosis trans-membrane conductance regulator to the cytoskeleton," The Journal of Biological Chemistry 273(31)(Jul. 31 1998): 19797-19801. PMID 9677412.
- ↑ V. Pai, and M. Nahata, "Efficacy and safety of aerosolized tobramycin in cystic fibrosis," Pediatric Pulmonology 32(4)(Oct. 2001): 314-27. Review. PMID 11568993.
- ↑ E. Westerman, P. Le Brun, D. Touw, H. Frijlink, and H. Heijerman, "Effect of nebulized colistin sulphate and colistin sulphomethate on lung function in patients with cystic fibrosis: A pilot study," Journal of cystic fibrosis 3(1)(Mar. 2004): 23-8. PMID 15463883.
- ↑ C. Hansen, T. Pressler, C. Koch, and N. Hoiby, "Long-term azithromycin treatment of cystic fibrosis patients with chronic Pseudomonas aeruginosa infection; an observational cohort study," Journal of Cystic Fibrosis 4(1)(Mar. 2005): 35–40. PMID 15752679.
- ↑ C. van der Schans, A. Prasad, and E. Main, "Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis," Cochrane Database Syst Rev (2)(2000): CD001401. Review. PMID 10796781.
- ↑ R. Kuver, and S. P. Lee, "Hypertonic saline for cystic fibrosis," The New England Journal of Medicine 354(17)(Apr. 27 2006): 1848–51; author reply 1848–51. PMID 16642591.
- ↑ J. Lieberman, "Dornase aerosol effect on sputum viscosity in cases of cystic fibrosis," JAMA: The Journal of the American Medical Association 205(5)(Jul. 29 1968): 312-3. PMID 5694947.
- ↑ S. H. Donaldson et al., "Mucus clearance and lung function in cystic fibrosis with hypertonic saline," New England Journal of Medicine 354(3)(2006): 241-250. PMID 16421365.
- ↑ F. Moran, and J. Bradley, "Non-invasive ventilation for cystic fibrosis," Cochrane Database Syst Rev (2)(2003): CD002769. Review. PMID 12804435.
- ↑ G. Onady and A. Stolfi, "Insulin and oral agents for managing cystic fibrosis-related diabetes," Cochrane Database Syst Rev (3)(Jul. 20 2005): CD004730. Review. PMID 16034943.
- ↑ S. Conway, B. Oldroyd, A. Morton, J. Truscott, and D. Peckham, "Effect of oral bisphosphonates on bone mineral density and body composition in adult patients with cystic fibrosis: A pilot study," Thorax 59(8)(Aug. 2004): 699–703. PMID 15282392.
- ↑ D. Hardin, J. Rice, C. Ahn, T. Ferkol, M. Howenstine, S. Spears, C. Prestidge, D. Seilheimer, and R. Shepherd, "Growth hormone treatment enhances nutrition and growth in children with cystic fibrosis receiving enteral nutrition," The Journal of Pediatrics 146(3)(Mar. 2005): 324-328. PMID 15756212.
- ↑ S. Marks, and D. Kissner, "Management of sinusitis in adult cystic fibrosis," American Journal of Rhinology 11(1)(Jan.-Feb. 1997): 11-4. PMID 9065342.
- ↑ G. Phillipson, O. Petrucco, and C. Matthews, "Congenital bilateral absence of the vas deferens, cystic fibrosis mutation analysis and intracytoplasmic sperm injection," Human Reproduction 15(2)(Feb. 2000): 431-435. PMID 10655317.
- ↑ Fridell, J. A., R. Vianna, P. Y. Kwo, M. Howenstine, A. Sannuti, J. P. Molleston, M. D. Pescovitz, and A. J. Tector, "Simultaneous liver and pancreas transplantation in patients with cystic fibrosis," Transplantation proceedings 37(8) (Oct. 2005): 3567–9. PMID 16298663.
- ↑ R. Belkin, N. Henig, L. Singer, C. Chaparro, R. Rubenstein, S. Xie, J. Yee, R. Kotloff, D. Lipson, and G. Bunin, "Risk factors for death of patients with cystic fibrosis awaiting lung transplantation," American Journal of Respiratory and Critical Care Medicine 173(6)(Mar. 15 2006): 659-666. Epub 2005 Dec 30. PMID 16387803.
- ↑ A. Ramalho, S. Beck, M. Meyer, D. Penque, G. Cutting, and M. Amaral, "Five percent of normal cystic fibrosis transmembrane conductance regulator mRNA ameliorates the severity of pulmonary disease in cystic fibrosis," American Journal of Respiratory Cell and Molecular Biology 27(5)(Nov. 2002): 619-627. PMID 12397022.
- ↑ S. Tate, and S. Elborn, "Progress towards gene therapy for cystic fibrosis," Expert Opinion on Drug Delivery 2(2)(Mar. 2005): 269-80. Review. PMID 16296753.
- ↑ B. Rosenstein, and G. Cutting, "The diagnosis of cystic fibrosis: A consensus statement. Cystic Fibrosis Foundation Consensus Panel," The Journal of Pediatrics 132(4)(Apr. 1998): 589-95. Review. PMID 9580754.
- ↑ A. Hamosh, S. Fitz-Simmons, M. Macek Jr., M. Knowles, B. Rosenstein, and G. Cutting, "Comparison of the clinical manifestations of cystic fibrosis in black and white patients," The Journal of Pediatrics 132(2)(Feb. 1998): 255-259. PMID 9506637.
- ↑ B. Kerem, O. Chiba-Falek, and E. Kerem, "Cystic fibrosis in Jews: Frequency and mutation distribution," Genetic Testing 1(1)(1997): 35-9. Review. PMID 10464623.
- ↑ M. Rosenfeld, R. Davis, S. Fitz-Simmons, et. al., "Gender gap in cystic fibrosis mortality," American Journal of Epidemiology 145(1997): 794–803.
- ↑ Cystic Fibrosis Foundation, New statistics show CF patients living longer, Cystic Fibrosis Foundation. Retrieved November 7, 2007.
- ↑ C. Wiuf, "Do delta F508 heterozygotes have a selective advantage?" Genetical Research 78(1)(Aug. 2001): 41-7. PMID 11556136.
- ↑ S. Gabriel, K. Brigman, B. Koller, R. Boucher, and M. Stutts, "Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model," Science 266(5182)(Oct. 7 1994): 107-9. PMID 7524148.
- ↑ A. Cuthbert, J. Halstead, R. Ratcliff, W. Colledge, and M. Evans, "The genetic advantage hypothesis in cystic fibrosis heterozygotes: A murine study," The Journal of Physiology 482 (Pt 2)(Jan. 15 1995): 449-54. PMID 7714835.
- ↑ C. Hogenauer, C. Santa Ana, J. Porter, M. Millard, A. Gelfand, R. Rosenblatt, C. Prestidge, and J. Fordtran, "Active intestinal chloride secretion in human carriers of cystic fibrosis mutations: An evaluation of the hypothesis that heterozygotes have subnormal active intestinal chloride secretion," American Journal of Human Genetics 67(6)(Dec. 2000): 1422–7. Epub 2000 Oct 30. PMID 11055897.
- ↑ G. Pier, M. Grout, T. Zaidi, G. Meluleni, S. Mueschenborn, G. Banting, R. Ratcliff, M. Evans, and W. Colledge, "Salmonella typhi uses CFTR to enter intestinal epithelial cells," Nature 393(6680)(May 7 1998): 79–82. PMID 9590693.
- ↑ G. Modiano, B. Ciminelli, and P. Pignatti, "Cystic Fibrosis: Cystic fibrosis and lactase persistence: A possible correlation," European Journal of Human Genetics 15(3)(Mar. 2007): 255-9. PMID: 17180122.
- ↑ MacKenzie, D., Cystic fibrosis gene protects against tuberculosis, NewScientist (2006). Retrieved November 7, 2007.
- ↑ N. Williams, Footprint fears for new TB threat, Elsevier Article Locator (2003). Retrieved November 7, 2007.
- ↑ R. Busch, "On the history of cystic fibrosis," Acta Universitatis Carolinae. Medica. (Praha) 36(1-4)(1990): 13-5. PMID 2130674.
- ↑ G. Fanconi, E. Uehlinger, and C. Knauer, "Das coeliakiesyndrom bei angeborener zysticher pankreasfibromatose und bronchiektasien," Wiener medizinische Wochenschrift 86(1936): 753–756.
- ↑ D. Andersen, "Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathological study," American Journal of Diseases of Children 56(1938): 344–399.
- ↑ P. Di Sant' Agnese, R. Darling, G. Perera, et al., "Abnormal electrolyte composition of sweat in cystic fibrosis of the pancreas: clinical implications and relationship to the disease," Pediatrics 12(1953): 549–563.
- ↑ J. Riordan, J. Rommens, B. Kerem, N. Alon, R. Rozmahel, Z. Grzelczak, J. Zielenski, S. Lok, N. Plavsic, J. Chou, et al., "Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA," Science 245(4922)(Sep. 8 1998): 1066–73. Erratum in: Science 245(4925) (Sep. 29 1989): 1437. PMID 2475911.
- ↑ J. Rommens, M. Iannuzzi, B. Kerem, M. Drumm, G. Melmer, M. Dean, R. Rozmahel, J. Cole, D. Kennedy, N. Hidaka, et al., "Identification of the cystic fibrosis gene: chromosome walking and jumping," Science 245(4922)(Sep. 8 1989): 1059–65. PMID 2772657.
- ↑ Cystic Fibrosis Foundation, About 65 roses, Cystic Fibrosis Foundation (2007). Retrieved November 7, 2007.
- ↑ Tele News, L'actualité citoyenne, Telenews (2007). In French. Retrieved November 7, 2007.
External links
All links retrieved June 23, 2022.
| ||||||||||||||||||||||||||||||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.