Diamond
- This article is about the mineral.
| Diamond | |
|---|---|
 A scattering of round-brilliant cut diamonds shows off the many reflecting facets. |
|
| General | |
| Category | Native Minerals |
| Chemical formula | C |
| Identification | |
| Molecular Weight | 12.01 u |
| Color | Typically yellow, brown or gray to colorless. Less often in blue, green, black, translucent white, pink, violet, orange, purple and red.[1] |
| Crystal habit | Octahedral |
| Crystal system | Isometric-Hexoctahedral (Cubic) |
| Cleavage | 111 (perfect in four directions) |
| Fracture | Conchoidal - step like |
| Mohs Scale hardness | 10[1] |
| Luster | Adamantine[1] |
| Refractive index | 2.4175–2.4178 |
| Optical Properties | Singly Refractive[1] |
| Birefringence | none[1] |
| Pleochroism | none[1] |
| Streak | Colorless |
| Specific gravity | 3.52 (+/- .01)[1] |
| Density | 3.5-3.53 |
| Diaphaneity | Transparent to subtransparent to translucent |
Diamond is the hardest known natural material and the third-hardest known material after aggregated diamond nanorods and ultrahard fullerite. Its hardness and high dispersion of light make it useful for industrial applications and jewelry.
Diamonds are specifically renowned as a material with superlative physical qualities—they make excellent abrasives because they can be scratched only by other diamonds, Borazon, ultrahard fullerite, or aggregated diamond nanorods. This also means that they hold a polish extremely well and retain their luster.
The name diamond derives from the ancient Greek adamas (αδάμας), meaning "invincible" or "unbreakable." Diamonds have been treasured as gemstones since their use as religious icons in ancient India. Their usage in engraving tools also dates to early human history.[2][3] The popularity of diamonds has risen since the nineteenth century because of increased supply, improved cutting and polishing techniques, growth in the world economy, and innovative and successful advertising campaigns. They are commonly judged by the “four Cs”: carat, clarity, color, and cut.
Diamond crystals are formed deep within the Earth, under conditions of high pressure and high temperature. They are then brought to the surface in kimberlite and lamproite volcanic pipes, from which they are mined. Roughly 49 percent of diamonds originate from central and southern Africa, although significant sources of the mineral have been discovered in Canada, India, Russia, Brazil, and Australia.
The attractiveness and high prices of natural diamonds have also brought out the darker of human nature, such as theft, violence, and the sale of conflict diamonds by African paramilitary groups.
Formation
The formation of natural diamond requires very specific conditions. Diamond formation requires exposure of carbon-bearing materials to high pressure, ranging approximately between 45 and 60 kilobars, but at a comparatively low temperature range between approximately 1652–2372 °F (900–1300 °C).[4] These conditions are known to be met in two places on Earth; in the lithospheric mantle below relatively stable continental plates, and at the site of a meteorite strike.
Diamonds formed in cratons
The conditions for diamond formation to happen in the lithospheric mantle occur at considerable depth corresponding to the aforementioned requirements of temperature and pressure. These depths are estimated to be in between 90–120 miles (140–190 kilometers)[4][5] though occasionally diamonds have crystallized at depths of 300-400 km as well.[6] The rate at which temperature changes with increasing depth into the Earth varies greatly in different parts of the Earth. In particular, under oceanic plates the temperature rises more quickly with depth, beyond the range required for diamond formation at the depth required.[4] The correct combination of temperature and pressure is only found in the thick, ancient, and stable parts of continental plates where regions of lithosphere known as cratons exist.[4] Long residence in the cratonic lithosphere allows diamond crystals to grow larger.
Through studies of carbon isotope ratios (similar to the methodology used in carbon dating, except with the stable isotopes C-12 and C-13), it has been shown that the carbon found in diamonds comes from both inorganic and organic sources. Some diamonds, known as harzburgitic, are formed from inorganic carbon originally found deep in the Earth's mantle. In contrast, eclogitic diamonds contain organic carbon from organic detritus that has been pushed down from the surface of the Earth's crust through subduction (see plate tectonics) before transforming into diamond.[5] These two different source carbons have measurably different 13C:12C ratios. Diamonds that have come to the Earth's surface are generally very old, ranging from under 1 billion to 3.3 billion years old.
Diamonds occur most often as euhedral or rounded octahedra and twinned octahedra known as macles or maccles. As diamond's crystal structure has a cubic arrangement of the atoms, they have many facets that belong to a cube, octahedron, rhombicosidodecahedron, tetrakis hexahedron or disdyakis dodecahedron. The crystals can have rounded off and unexpressive edges and can be elongated. Sometimes they are found grown together or form double "twinned" crystals grown together at the surfaces of the octahedron. These different shapes and habits of the diamonds result from differing external circumstances. Diamonds (especially those with rounded crystal faces) are commonly found coated in nyf, an opaque gum-like skin.[7]
Diamonds and meteorite strikes
Diamonds can also form in other natural high-pressure, relatively low-temperature events. Very small diamonds, known as microdiamonds or nanodiamonds, have been found in impact craters where meteors strike the Earth and create shock zones of high pressure and temperature where diamond formation can occur. Microdiamonds are now used as one indicator of ancient meteorite impact sites.[5]
Surfacing
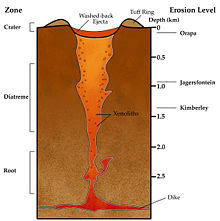
Diamond-bearing rock is brought close to the surface through deep-origin volcanic eruptions. The magma for such a volcano must originate at a depth where diamonds can be formed,[5] 150 km (90 miles) deep or more (three times or more the depth of source magma for most volcanoes); this is a relatively rare occurrence. These typically small surface volcanic craters extend downward in formations known as volcanic pipes.[5] The pipes contain material that was transported toward the surface by volcanic action, but was not ejected before the volcanic activity ceased. During eruption these pipes are open to the surface, resulting in open circulation; many xenoliths of surface rock and even wood and/or fossils are found in volcanic pipes. Diamond-bearing volcanic pipes are closely related to the oldest, coolest regions of continental crust (cratons). This is because cratons are very thick, and their lithospheric mantle extends to great enough depth that diamonds are stable. Not all pipes contain diamonds, and even fewer contain enough diamonds to make mining economically viable.
The magma in volcanic pipes is usually one of two characteristic types, which cool into igneous rock known as either kimberlite or lamproite.[5] The magma itself does not contain diamond; instead, it acts as an elevator that carries deep-formed rocks (xenoliths), minerals (xenocrysts), and fluids upward. These rocks are characteristically rich in magnesium-bearing olivine, pyroxene, and amphibole minerals[5] which are often altered to serpentine by heat and fluids during and after eruption. Certain indicator minerals typically occur within diamondiferous kimberlites and are used as mineralogic tracers by prospectors, who follow the indicator trail back to the volcanic pipe which may contain diamonds. These minerals are rich in chromium (Cr) or titanium (Ti), elements which impart bright colors to the minerals. The most common indicator minerals are chromian garnets (usually bright red Cr-pyrope, and occasionally green ugrandite-series garnets), eclogitic garnets, orange Ti-pyrope, red high-Cr spinels, dark chromite, bright green Cr-diopside, glassy green olivine, black picroilmenite, and magnetite.[5] Kimberlite deposits are known as blue ground for the deeper serpentinized part of the deposits, or as yellow ground for the near surface smectite clay and carbonate weathered and oxidized portion.
Once diamonds have been transported to the surface by magma in a volcanic pipe, they may erode out and be distributed over a large area. A volcanic pipe containing diamonds is known as a primary source of diamonds. Secondary sources of diamonds include all areas where a significant number of diamonds, eroded out of their kimberlite or lamproite matrix, accumulate because of water or wind action. These include alluvial deposits and deposits along existing and ancient shorelines, where loose diamonds tend to accumulate because of their approximate size and density. Diamonds have also rarely been found in deposits left behind by glaciers (notably in Wisconsin and Indiana); however, in contrast to alluvial deposits, glacial deposits are not known to be of significant concentration and are therefore not viable commercial sources of diamond.
Diamonds can also be brought to the surface through certain processes which may occur when two continental plates collide and deeply formed rock is thrust to the surface, although this phenomenon is less understood and currently assumed to be uncommon.
Material properties
A diamond is a transparent crystal of tetrahedrally bonded carbon atoms and crystallizes into the face centered cubic diamond lattice structure. Diamonds have been adapted for many uses because of the material's exceptional physical characteristics. Most notable are its extreme hardness, its high dispersion index, and extremely high thermal conductivity (900 – 2320 W/m K), with a melting point of 3820 K (3547 °C / 6420 °F) and a boiling point of 5100 K (4827 °C / 8720 °F).[8] Naturally occurring diamond has a density ranging from 3.15 to 3.53 g/cm³, with very pure diamond typically extremely close to 3.52 g/cm³.
Hardness
Diamond is the hardest natural material known to man - its hardness set to 10 (hardest) on Mohs scale of mineral hardness[5] and having an absolute hardness value of between 90, 167, and 231 gigapascals in various tests. Diamond's hardness has been known since antiquity, and is the source of its name. However, aggregated diamond nanorods, an allotrope of carbon synthesized by compressing fullerene, are even harder than diamond.[9]
The hardest diamonds in the world are from the New England area in New South Wales, Australia. These diamonds are generally small, perfect to semiperfect octahedra, and are used to polish other diamonds. Their hardness is considered to be a product of the crystal growth form, which is single stage growth crystal. Most other diamonds show more evidence of multiple growth stages, which produce inclusions, flaws, and defect planes in the crystal lattice, all of which affect their hardness.[10]
The hardness of diamonds contributes to its suitability as a gemstone. Because it can only be scratched by other diamonds, it maintains its polish extremely well, keeping its luster over long periods of time. Unlike many other gems, it is well-suited to daily wear because of its resistance to scratching—perhaps contributing to its popularity as the preferred gem in an engagement ring or wedding ring, which are often worn every day. Industrial use of diamonds has historically been associated with their hardness; this property makes diamond the ideal material for cutting and grinding tools. As the hardest known naturally occurring material, diamond can be used to polish, cut, or wear away any material, including other diamonds. However, diamond is a poor choice for machining ferrous alloys at high speeds. At the high temperatures created by high speed machining, carbon is soluble in iron, leading to greatly increased wear on diamond tools as compared to other alternatives. Common industrial adaptations of this ability include diamond-tipped drill bits and saws, or use of diamond powder as an abrasive. Industrial-grade diamonds are either unsuitable for use as gems or synthetically produced, which lowers their value and makes their use economically feasible. Industrial applications, especially as drill bits and engraving tools, also date to ancient times.
Electrical conductivity
Other specialized applications also exist or are being developed, including use as semiconductors: some blue diamonds are natural semiconductors, in contrast to most other diamonds, which are excellent electrical insulators.[5]
Toughness
Toughness relates to a material's ability to resist breakage from forceful impact. The toughness of natural diamond has been measured as 3.4 MN m-3/2,[11] which is good compared to other gemstones, but poor compared to most engineering materials. As with any material, the macroscopic geometry of a diamond contributes to its resistance to breakage. Diamond is therefore more fragile in some orientations than others.
Color
Diamonds can occur in nearly any color, though yellow and brown are by far the most common.[5] "Black" diamonds are not truly black, but rather contain numerous dark inclusions that give the gems their dark appearance. When the color is saturated enough in yellow or brown diamonds, a stone may be referred to as a fancy colored diamond by the gem trade, otherwise they are graded for color in the normal color range of white diamonds. Colored diamonds contain impurities or structural defects that cause the coloration, while pure or nearly pure diamonds are transparent and colorless. Most diamond impurities replace a carbon atom in the crystal lattice, known as a carbon flaw. The most common impurity, nitrogen, causes a slight to intense yellow coloration depending upon the type and concentration of nitrogen present.[5] The Gemological Institute of America (GIA) classifies low saturation yellow and brown diamonds as diamonds in the normal color range, and applies a grading scale from 'D' (colorless) to 'Z' (light yellow). The GIA labels diamonds that have more color than a 'Z' diamond "fancy," along with those that are any color other than yellow or brown.
Identification
Diamonds can be identified via their high thermal conductivity. Their high refractive index is also indicative, but other materials have similar refractivity. Diamonds do cut glass, but other materials above glass on Mohs scale such as quartz do also. Diamonds can scratch other diamonds, but this damages both diamonds.
Gemological characteristics
The most familiar usage of diamonds today is as gemstones used for adornment. This usage dates back into antiquity and predates other uses. The dispersion of white light into spectral colors, is the primary gemological characteristic of gem diamonds. In the twentieth century, experts in the field of gemology have developed methods of grading diamonds and other gemstones based on the characteristics most important to their value as a gem. Four characteristics, known informally as the four Cs, are now commonly used as the basic descriptors of diamonds: these are carat, cut, color, and clarity.
Most gem diamonds are traded on the wholesale market based on single values for each of the four Cs; for example knowing that a diamond is rated as 1.5 carats (300 mg), VS2 clarity, F color, excellent cut round brilliant, is enough to reasonably establish an expected price range. More detailed information from within each characteristic is used to determine actual market value for individual stones. Consumers who purchase individual diamonds are often advised to use the four Cs to pick the diamond that is "right" for them.
Other characteristics not described by the four Cs influence the value or appearance of a gem diamond. These characteristics include physical characteristics such as the presence of fluorescence, as well as data on a diamond's history including its source and which gemological institute performed evaluation services on the diamond. Cleanliness also dramatically affects a diamond's beauty.
There are three major non-profit gemological associations which grade and provide reports on diamonds. While carat weight and cut angles are mathematically defined, the clarity and color are judged by the trained human eye and are therefore open to slight variance in interpretation.
- Gemological Institute of America (GIA) was the first laboratory in America to issue modern diamond reports, and is held in high regard among gemologists for its consistent, conservative grading.[12]
- American Gemological Society (AGS) is not as widely recognized nor as old as the GIA but garners a high reputation. The AGS employs a number system for grading cut quality, color grade, and clarity. The highest grade being '0', and the lowest being '10'.
- Diamond High Council (HRD) Official certification laboratory of the Belgian diamond industry, located in Antwerp.[13]
Carat
The carat weight measures the mass of a diamond. One carat is defined as 200 milligrams (about 0.007 ounce avoirdupois). The point unit—equal to one one-hundredth of a carat (0.01 carat, or 2 mg)—is commonly used for diamonds of less than one carat. All else being equal, the price per carat increases with carat weight, since larger diamonds are both rarer and more desirable for use as gemstones.
The price per carat does not increase smoothly with increasing size. Instead, there are sharp jumps around milestone carat weights, as demand is much higher for diamonds weighing just more than a milestone than for those weighing just less. As an example, a 0.95 carat diamond may have a significantly lower price per carat than a comparable 1.05 carat diamond, because of differences in demand.
A weekly diamond price list, the Rapaport Diamond Report is published by Martin Rapaport, CEO of Rapaport Group of New York, for different diamond cuts, clarity and weights.[14] It is currently considered the de-facto retail price baseline. Jewelers often trade diamonds at negotiated discounts off the Rapaport price (e.g., "R -3%").
In the wholesale trade of gem diamonds, carat is often used in denominating lots of diamonds for sale. For example, a buyer may place an order for 100 carats of 0.5 carat, D–F, VS2-SI1, excellent cut diamonds, indicating he wishes to purchase 200 diamonds (100 carats total mass) of those approximate characteristics. Because of this, diamond prices (particularly among wholesalers and other industry professionals) are often quoted per carat, rather than per stone.
Total carat weight (t.c.w.) is a phrase used to describe the total mass of diamonds or other gemstone in a piece of jewelry, when more than one gemstone is used. Diamond solitaire earrings, for example, are usually quoted in t.c.w. when placed for sale, indicating the mass of the diamonds in both earrings and not each individual diamond. T.c.w. is also widely used for diamond necklaces, bracelets and other similar jewelry pieces.
Clarity
Clarity is a measure of internal defects of a diamond called inclusions. Inclusions may be crystals of a foreign material or another diamond crystal, or structural imperfections such as tiny cracks that can appear whitish or cloudy. The number, size, color, relative location, orientation, and visibility of inclusions can all affect the relative clarity of a diamond. The Gemological Institute of America (GIA) and other organizations have developed systems to grade clarity, which are based on those inclusions which are visible to a trained professional when a diamond is viewed under 10x magnification.
Diamonds become increasingly rare when considering higher clarity gradings. Only about 20 percent of all diamonds mined have a clarity rating high enough for the diamond to be considered appropriate for use as a gemstone; the other 80 percent are relegated to industrial use. Of that top 20 percent, a significant portion contains one or more visible inclusions. Those that do not have a visible inclusion are known as "eye-clean" and are preferred by most buyers, although visible inclusions can sometimes be hidden under the setting in a piece of jewelry.
Most inclusions present in gem-quality diamonds do not affect the diamonds' performance or structural integrity. However, large clouds can affect a diamond's ability to transmit and scatter light. Large cracks close to or breaking the surface may reduce a diamond's resistance to fracture.
Diamonds are graded by the major societies on a scale ranging from flawless to imperfect.
Color

A chemically pure and structurally perfect diamond is perfectly transparent with no hue, or color. However, in reality almost no gem-sized natural diamonds are absolutely perfect. The color of a diamond may be affected by chemical impurities and/or structural defects in the crystal lattice. Depending on the hue and intensity of a diamond's coloration, a diamond's color can either detract from or enhance its value. For example, most white diamonds are discounted in price as more yellow hue is detectable, while intense pink or blue diamonds (such as the Hope Diamond) can be dramatically more valuable. The Aurora Diamond Collection displays a spectacular array of naturally colored diamonds.
Most diamonds used as gemstones are basically transparent with little tint, or white diamonds. The most common impurity, nitrogen, replaces a small proportion of carbon atoms in a diamond's structure and causes a yellowish to brownish tint. This effect is present in almost all white diamonds; in only the rarest diamonds is the coloration from this effect undetectable. The GIA has developed a rating system for color in white diamonds, from "D" to "Z" (with D being "colorless" and Z having a bright yellow coloration), which has been widely adopted in the industry and is universally recognized, superseding several older systems once used in different countries. The GIA system uses a benchmark set of natural diamonds of known color grade, along with standardized and carefully controlled lighting conditions. Precision-crafted cubic zirconia master sets are sometimes used in the trade, however the GIA has found these sets to be inaccurate. Diamonds with higher color grades are rarer, in higher demand, and therefore more expensive, than lower color grades. Oddly enough, diamonds graded Z are also rare, and the bright yellow color is also highly valued. Diamonds graded D-F are considered "colorless," G-J are considered "near-colorless," K-M are "slightly colored." N-Y usually appear light yellow or brown.
In contrast to yellow or brown hues, diamonds of other colors are more rare and valuable. While even a pale pink or blue hue may increase the value of a diamond, more intense coloration is usually considered more desirable and commands the highest prices. A variety of impurities and structural imperfections cause different colors in diamonds, including yellow, pink, blue, red, green, brown, and other hues. Diamonds with unusual or intense coloration are sometimes labeled "fancy" by the diamond industry. Intense yellow coloration is considered one of the fancy colors, and is separate from the color grades of white diamonds. Gemologists have developed rating systems for fancy colored diamonds, but they are not in common use because of the relative rarity of colored diamonds.
Cut
Diamond cutting is the art and science of creating a gem-quality diamond out of mined rough. The cut of a diamond describes the manner in which a diamond has been shaped and polished from its beginning form as a rough stone to its final gem proportions. The cut of a diamond describes the quality of workmanship and the angles to which a diamond is cut. Often diamond cut is confused with "shape."
There are mathematical guidelines for the angles and length ratios at which the diamond is supposed to be cut in order to reflect the maximum amount of light. Round brilliant diamonds, the most common, are guided by these specific guidelines, though fancy cut stones are not able to be as accurately guided by mathematical specifics.
The techniques for cutting diamonds have been developed over hundreds of years, with perhaps the greatest achievements made in 1919 by mathematician and gem enthusiast Marcel Tolkowsky. He developed the round brilliant cut by calculating the ideal shape to return and scatter light when a diamond is viewed from above. The modern round brilliant has 57 facets (polished faces), counting 33 on the crown (the top half), and 24 on the pavilion (the lower half). The girdle is the thin middle part. The function of the crown is to diffuse light into various colors and the pavilion's function to reflect light back through the top of the diamond.
Tolkowsky defines the ideal dimensions to have:
- Table percentage (table diameter divided by overall diameter) = 53 percent
- Depth percentage (Overall depth divided by the overall diameter) = 59.3 percent
- Pavilion Angle (Angle between the girdle and the pavilion) = 40.75°
- Crown Angle (Angle between the girdle and the crown) = 34.5°
- Pavilion Depth (Depth of pavilion divided by overall diameter) = 43.1 percent
- Crown Depth (Depth of crown divided by crown diameter) = 16.2 percent
The culet is the tiny point or facet at the bottom of the diamond. This should be a negligible diameter, otherwise light leaks out of the bottom. Tolkowsky's ideal dimensions did not include a culet. However, a thin culet is required in reality in order to prevent the diamond from easily chipping in the setting. A normal culet should be about 1–2 percent of the overall diameter.
The further the diamond's characteristics are from Tolkowsky's ideal, the less light will be reflected. However, there is a small range in which the diamond can be considered "ideal." Today, because of the relative importance of carat weight in society, many diamonds are often intentionally cut poorly to increase carat weight. There is a financial premium for a diamond that weighs the magical 1.0 carat, so often the girdle is made thicker or the depth is increased. Neither of these tactics make the diamond appear any bigger, and they greatly reduce the sparkle of the diamond. So a poorly cut 1.0 carat diamond may have the same diameter and appear as large as a 0.85 carat diamond. The depth percentage is the overall quickest indication of the quality of the cut of a round brilliant. "Ideal" round brilliant diamonds should not have a depth percentage greater than 62.5%. Another quick indication is the overall diameter. Typically a round brilliant 1.0 carat diamond should have a diameter of about 6.5 mm. Mathematically, the diameter in millimeters of a round brilliant should approximately equal 6.5 times the cube root of carat weight, or 11.1 times the cube root of gram weight, or 1.4 times the cube root of point weight.
Ideal cuts can be controversial as the definitions of brilliance and beauty are very subjective.
Tolkowsky's mathematical model is now superseded by the GIA Facetware software that is the culmination of 20 years of studies on diamond cuts.
New diamond cuts are now all the rage in the diamond industry as for example a design invented in 2003 and called the "Genesis cut." This cut differs in shape from the more traditional cuts in its concave surfaces and angles and resembles a 4-pointed star.
Shape
Diamonds do not show all of their beauty as rough stones; instead, they must be cut and polished to exhibit the characteristic fire and brilliance that diamond gemstones are known for. Diamonds are cut into a variety of shapes that are generally designed to accentuate these features.
Diamonds which are not cut to the specifications of Tolkowsky's round brilliant shape (or subsequent variations) are known as "fancy cuts." Popular fancy cuts include the baguette (from the French, meaning rod or loaf of bread), marquise, princess cut (square outline), heart, briolette (a form of the rose cut), and pear cuts. Newer cuts that have been introduced into the jewelry industry are the "cushion" "radiant"(similar to princess cuts, but with rounded edges instead of square edges) and "Asscher" cuts. Many fancy colored diamonds are now being cut according to these new styles. Generally speaking, these "fancy cuts" are not held to the same strict standards as Tolkowsky-derived round brilliants and there are less specific mathematical guidelines of angles which determine a well-cut stone. Cuts are influenced heavily by fashion: the baguette cut—which accentuates a diamond's luster and downplays its fire—was all the rage during the Art Deco period, whereas the princess cut cut—which accentuates a diamond's fire rather than its luster—is currently gaining popularity. The princess cut is also popular amongst diamond cutters: of all the cuts, it wastes the least of the original crystal. The past decades have seen the development of new diamond cuts, often based on a modification of an existing cut. Some of these include extra facets. These newly developed cuts are viewed by many as more of an attempt at brand differentiation by diamond sellers, than actual improvements to the state of the art.
Quality
The quality of a diamond's cut is widely considered the most important of the four Cs in determining the beauty of a diamond; indeed, it is commonly acknowledged that a well-cut diamond can appear to be of greater carat weight, and have clarity and color appear to be of better grade than they actually are. The skill with which a diamond is cut determines its ability to reflect and refract light.
In addition to carrying the most importance to a diamond's quality as a gemstone, the cut is also the most difficult to quantitatively judge. A number of factors, including proportion, polish, symmetry, and the relative angles of various facets, are determined by the quality of the cut and can affect the performance of a diamond. A poorly cut diamond with facets cut only a few degrees out of alignment can result in a poorly performing stone. For a round brilliant cut, there is a balance between "brilliance" and "fire." When a diamond is cut for too much "fire," it looks like a cubic zirconia, which gives off much more "fire" than real diamond. A well-executed round brilliant cut should reflect light upwards and make the diamond appear white when viewed from the top. An inferior cut will produce a stone that appears dark at the center and in some extreme cases the ring settings may show through the top of the diamond as shadows.
Several different theories on the "ideal" proportions of a diamond have been and continue to be advocated by various owners of patents on machines to view how well a diamond is cut. These advocate a shift away from grading cut by the use of various angles and proportions toward measuring the performance of a cut stone. A number of specially modified viewers and machines have been developed toward this end. Hearts and Arrows viewers test for the "hearts and arrows" characteristic pattern observable in stones exhibiting high symmetry and particular cut angles. Closely related to Hearts and Arrows viewers is the Angular Spectrum Evaluation Tool (ASET) which tests for light leakage, light return, and proportions.[15] The ASET (and computer simulations of the ASET) are used to test for AGS cut grade. These viewers and machines often help sellers demonstrate the light performance results of the diamond in addition to the traditional 4 Cs. Detractors see these machines as marketing tools rather than as scientific tools.
The GIA has developed a set of criteria for grading the cut of round brilliant stones that is now the standard in the diamond industry and is called "Facetware."
Process
The process of shaping a rough diamond into a polished gemstone is both an art and a science. The choice of cut is often decided by the original shape of the rough stone, location of the inclusions and flaws to be eliminated, the preservation of the weight, popularity of certain shapes amongst consumers and many other considerations. The round brilliant cut is preferred when the crystal is an octahedron, as often two stones may be cut from one such crystal. Oddly shaped crystals are more likely to be cut in a fancy cut—that is, a cut other than the round brilliant—which the particular crystal shape lends itself to.
Even with modern techniques, the cutting and polishing of a diamond crystal always results in a dramatic loss of weight; rarely is it less than 50 percent. Sometimes the cutters compromise and accept lesser proportions and symmetry in order to avoid inclusions or to preserve the carat rating. Since the per carat price of diamond shifts around key milestones (such as 1.00 carat), many one-carat diamonds are the result of compromising "Cut" for "Carat." Some jewelry experts advise consumers to buy a 0.99 carat diamond for its better price or buy a 1.10 carat diamond for its better cut, avoiding a 1.00 carat diamond which is more likely to be a poorly cut stone.
Light performance
In the gem trade, the term light performance is used to describe how well a polished diamond will return light to the viewer. There are three light properties which are described in relation to light performance; brilliance, fire, and scintillation. Brilliance refers to the white light reflections from the external and internal facet surfaces. Fire refers to the spectral colors which are produced as a result of the diamond dispersing the white light. Scintillation refers to the small flashes of light that are seen when the diamond, light source or the viewer is moved. A diamond that is cut and polished to produce a high level of these qualities is said to be high in light performance.
The setting diamonds are placed in also affect the performance of light through a diamond. The three most commonly used settings are: Prong, Bezel, and Channel. Prong settings are the most popular setting for diamond jewelry. The prong setting consists of four or six 'claws' that cradle the diamond, allowing the maximum amount of light to enter from all angles, allowing the diamonds to appear larger and more brilliant. In bezel settings the diamond or gemstone is completely surrounded by a rim of metal, which can be molded into any shape to accommodate the stone. Used to set earrings, necklaces, bracelets, and rings, bezel settings can have open or closed backs, and generally can be molded to allow a lot of light to pass through. Channel settings set the stones right next to each other with no metal separating them. This setting is mostly used in wedding and anniversary bands. The outer ridge is then worked over the edges of the stones to create a smooth exterior surface. This also protects the girdle area of the stone.
Fluorescence
About a third of all diamonds will glow under ultraviolet light, usually a blue color which may be noticeable under a black light or strong sunlight. According to the GIA, who reviewed a random sample of 26,010 natural diamonds, 65 percent of the diamonds in the sample had no fluorescence. Of the 35 percent that did have fluorescence, 97 percent had blue fluorescence of which 38 percent had faint blue fluorescence and 62 percent had fluorescence that ranged from medium to very strong blue. Other colors diamonds can fluoresce are green, yellow, and red but are very rare and are sometimes a combination of the colors such as blue-green or orange. Some diamonds with "very strong" fluorescence can have a "milky" or "oily" look to them, but they are also very rare and are termed "overblues." Their study concluded that with the exception of "overblues" and yellow fluorescent diamonds, fluorescence had little effect on transparency and that the strong and very strong blue fluorescent diamonds on average had better color appearance than non-fluorescent stones. Since blue is a complementary color to yellow and can appear to cancel it out, strong blue fluorescence had especially better color appearance with lower color graded diamonds that have a slight yellowish tint such as "I" color or "J" color but had little effect on the more colorless "D" through "F" color grades.[16]
Cleaning
Cleanliness heavily affects a diamond's beauty. A clean diamond is more brilliant and fiery than the same diamond when it is "dirty." Dirt or grease on the top of a diamond reduces its luster. Water, dirt, or grease on the bottom of a diamond interferes with the diamond's brilliance and fire. Even a thin film absorbs some light that could have been reflected to the person looking at the diamond. Colored dye or smudges can affect the perceived color of a diamond. Historically, some jewelers' stones were misgraded because of smudges on the girdle, or dye on the culet. Current practice is to clean a diamond thoroughly before grading its color.
Maintaining a clean diamond can sometimes be difficult as jewelry settings can obstruct cleaning efforts and oils, grease, and other hydrophobic materials adhere well to a diamond's surface. Many jewelers use steam cleaners. Some jewelers provide their customers with ammonia-based cleaning kits; ultrasonic cleaners are also popular.
History
Diamonds are thought to have been first recognized and mined in India (Golconda being one of them), where significant alluvial deposits of the stone could then be found along the rivers Penner, Krishna and Godavari. Diamonds have been known in India for at least 3000 years but most likely 6000 years.[17]
The earliest written reference can be found in the Buddhist text, the Anguttara Nikaya another Sanskrit text, the Arthashastra, which was completed around 296 B.C.E. and describes diamond's hardness, luster, and dispersion. Diamonds quickly became associated with divinity, being used to decorate religious icons, and were believed to bring good fortune to those who carried them. Ownership was restricted among various castes by color, with only kings being allowed to own all colors of diamond.
In February 2005, a joint Chinese-U.S. team of archaeologists reported the discovery of four corundum-rich stone ceremonial burial axes originating from China's Liangzhu and Sanxingcun cultures (4000 B.C.E.–2500 B.C.E.) which, because of the axes' specular surfaces, the scientists believe were polished using diamond powder.[3][18] Although there are diamond deposits now known to exist close to the burial sites, no direct evidence of coeval diamond mining has been found: the researchers came to this conclusion by polishing corundum using various lapidary abrasives and modern techniques then comparing the results using an atomic force microscope. At that scale, the surface of the modern diamond-polished corundum closely resembled that of the axes; however, the polishes of the latter were superior.
Diamonds were traded to both the east and west of India and were recognized by various cultures for their gemological or industrial uses. In his work Naturalis Historia, the Roman writer Pliny the Elder noted diamond's ornamental uses, as well as its usefulness to engravers because of its hardness. It is however highly doubtful that Pliny actually meant diamonds and it is assumed that in fact several different minerals such as Corundum, Spinel, or even a mixture with Magnetite were all referred to by the word "adamas."[19] In China, diamonds seem to have been used primarily as diamond tools for engraving jade and drilling holes in beads. Archaeological evidence from Yemen suggests that diamonds were used as drill tips as early as the fourth century B.C.E. In Europe, however, diamonds disappeared for almost 1000 years following the rise of Christianity because of two effects: early Christians rejected diamonds because of their earlier use in amulets, and Arabic traders restricted the flow of trade between Europe and India.
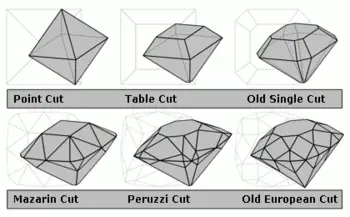
Until the late Middle Ages, diamonds were most prized in their natural octahedral state, perhaps with the crystal surfaces polished to increase luster and remove foreign material. Around 1300, the flow of diamonds into Europe increased via Venice's trade network, with most flowing through the low country ports of Bruges, Antwerp, and Amsterdam. During this time, the taboo against cutting diamonds into gem shapes, which was established over 1000 years earlier in the traditions of India, ended allowing the development of diamond cutting technology to begin in earnest. By 1375, a guild of diamond polishers had been established at Nuremberg. Over the following centuries, various diamond cuts were introduced which increasingly demonstrated the fire and brilliance that makes diamonds treasured today: the table cut, the briolette (around 1476), the rose cut (mid-sixteenth century), and by the mid-seventeenth century, the Mazarin, the first brilliant cut diamond design. In 1919, Marcel Tolkowsky developed an ideal round brilliant cut design that has set the standard for comparison of modern gems; however, diamond cuts have continued to be refined.
The rise in popularity of diamonds as gems seems to have paralleled increasing availability through European history. In the thirteenth century, King Louis IX of France established a law that only the king could own diamonds. However, within a century diamonds were popular gems among the moneyed aristocratic and merchant classes, and by 1477 had begun to be used rarely in wedding rings. Diamond wedding rings didn't gain widespread social significance until the De Beers company started marketing the idea through cinema beginning in the 1940s. A number of large diamonds have become historically significant objects, as their inclusion in various sets of crown jewels and the purchase, sale, and sometimes theft of notable diamonds, have sometimes become politicized.
Record-holding diamonds
The Cullinan Diamond, part of the British crown jewels, was the largest gem-quality rough diamond ever found (1905), at 3,106.75 carats. One of the diamonds cut from it, Cullinan I or the Great Star of Africa, was formerly the largest gem-quality cut diamond at 530.2 carats, but now that title has been taken by the Golden Jubilee (1985), a 545.67 carat, yellow-brown diamond. The largest flawless and colorless (grade D) diamond is the Centenary Diamond which weighs 273.85 carats. The Millennium Star is the second largest (1990) at 203.04 carats.
Travis Metcalfe, an astronomer at the Harvard-Smithsonian Center for Astrophysics, believes that the galaxy's largest diamond is the core of the white dwarf star BPM 37093. Observations indicate that the core is a diamond crystal 4000 km in diameter,[20] with a corresponding weight of over 5 × 1026 carats.
Stone of the Century
On August 28, 2007, a giant gem, 7,000 carats (2x the size of the Cullinan Diamond and about the size of a coconut, with a greenish tinge) was reported discovered by a small South African mining company. Still to be confirmed, Brett Jolly (shareholder at the mine), deposited the gem to a bank vault in Johannesburg.[21] The company announced that it asked the president of the World Federation of Diamond Bourses to make the examination next week. De Beers however stated that "the north-west province was not known for producing gems and greenish stones were even rarer."[22]
The diamond industry
The diamond industry can be broadly separated into two basically distinct categories: one dealing with gem-grade diamonds and another for industrial-grade diamonds. While a large trade in both types of diamonds exists, the two markets act in dramatically different ways.
Gem diamond industry
A large trade in gem-grade diamonds exists. Unlike precious metals such as gold or platinum, gem diamonds do not trade as a commodity: there is a substantial mark-up in the sale of diamonds, and there is not a very active market for resale of diamonds. One hallmark of the trade in gem-quality diamonds is its remarkable concentration: wholesale trade and diamond cutting is limited to a few locations (most importantly Antwerp, London, New York, Tel Aviv, Amsterdam and Surat), and a single company—De Beers—controls a significant proportion of the trade in diamonds. They are based in Johannesburg, South Africa and London, England.
The production and distribution of diamonds is largely consolidated in the hands of a few key players, and concentrated in traditional diamond trading centers. The most important being Antwerp, where 80% of all rough diamonds, 50% of all cut diamonds and more than 50% of all rough, cut and industrial diamonds combined are handled. This makes Antwerp the de facto 'world diamond capital'. New York, however, along with the rest of the United States, is where almost 80% of the world's diamonds are sold, including at auction. Also, the largest and most unusually shaped rough diamonds end up in New York. The De Beers company, as the world's largest diamond miner holds a clearly dominant position in the industry, and has done so since soon after its founding in 1888 by the British imperialist Cecil Rhodes. De Beers owns or controls a significant portion of the world's rough diamond production facilities (mines) and distribution channels for gem-quality diamonds. The company and its subsidiaries own mines that produce some 40 percent of annual world diamond production. At one time it was thought over 80 percent of the world's rough diamonds passed through the Diamond Trading Company (DTC, a subsidiary of De Beers) in London, but presently the figure is estimated at less than 50 percent.
The De Beers diamond advertising campaign is acknowledged as one of the most successful and innovative campaigns in history. N.W. Ayer & Son, the advertising firm retained by De Beers in the mid-20th century, succeeded in reviving the American diamond market and opened up new markets, even in countries where no diamond tradition had existed before. N.W. Ayer's multifaceted marketing campaign included product placement, advertising the diamond itself rather than the De Beers brand, and building associations with celebrities and royalty. This coordinated campaign has lasted decades and continues today; it is perhaps best captured by the slogan "a diamond is forever."
Further down the supply chain, members of The World Federation of Diamond Bourses (WFDB) act as a medium for wholesale diamond exchange, trading both polished and rough diamonds. The WFDB consists of independent diamond bourses in major cutting centers such as Tel Aviv, Antwerp, Johannesburg and other cities across the USA, Europe and Asia.
In 2000, the WFDB and The International Diamond Manufacturers Association established the World Diamond Council to prevent the trading of diamonds used to fund war and inhumane acts.
WFDB's additional activities also include sponsoring the World Diamond Congress every two years, as well as the establishment of the International Diamond Council (IDC) to oversee diamond grading.
Industrial diamond industry
The market for industrial-grade diamonds operates much differently from its gem-grade counterpart. Industrial diamonds are valued mostly for their hardness and heat conductivity, making many of the gemological characteristics of diamond, including clarity and color, mostly irrelevant. This helps explain why 80 percent of mined diamonds (equal to about 100 million carats or 20,000 kg annually), unsuitable for use as gemstones and known as bort, are destined for industrial use. In addition to mined diamonds, synthetic diamonds found industrial applications almost immediately after their invention in the 1950s.
The dominant industrial use of diamond is in cutting, drilling, grinding, and polishing. Most uses of diamonds in these technologies do not require large diamonds; in fact, most diamonds that are gem-quality except for their small size, can find an industrial use. Diamonds are embedded in drill tips or saw blades, or ground into a powder for use in grinding and polishing applications. Specialized applications include use in laboratories as containment for high pressure experiments (see diamond anvil), high-performance bearings, and limited use in specialized windows.
With the continuing advances being made in the production of synthetic diamonds, future applications are beginning to become feasible. Garnering much excitement is the possible use of diamond as a semiconductor suitable to build microchips from, or the use of diamond as a heat sink in electronics. Since the quality of the best synthetic diamonds is now equal to or better than that of the most perfect natural diamonds, significant changes in the gem-diamond industry may also be forthcoming.
Diamond supply chain
The diamond supply chain is controlled by a limited number of powerful businesses, and is also highly concentrated in a small number of locations around the world. In fact, the amount of power which De Beers has consolidated historically prevented it from direct trade with the United States, as its trade practices led to an indictment for violating antitrust regulations. The concentration of power only loosens at the retail level, where diamonds are sold by a limited number of distributors, known as sightholders, to jewelers around the world.

Sources
Historically, diamonds were known to be found only in alluvial deposits in southern India.[23] India led the world in diamond production from the time of their discovery in approximately the ninth century B.C.E.[24] to the mid-eighteenth century C.E., but the commercial potential of these sources has been exhausted by the late eighteenth century and at that time eclipsed by Brazil, and later South Africa.[17]
The first non-Indian diamond source was found in Brazil in 1725.[17] While no commercial diamond production exists in the U.S., Arkansas and Colorado are the only states to have a verifiable source of diamonds.[25]
Today, most commercially viable diamond deposits are in Russia, Botswana, Australia and the Democratic Republic of Congo. In 2005, Russia produced almost one-fifth of the global diamond output, reports the British Geological Survey. Australia boasts the richest diamondiferous pipe with production reaching peak levels of 42 Mct per year in the 1990s.[25]
There are also commercial deposits being actively mined in the Northwest Territories of Canada, Siberia (mostly in Yakutia territory, for example Mir pipe and Udachnaya pipe), Brazil, and in Northern and Western Australia. Diamond prospectors continue to search the globe for diamond-bearing kimberlite and lamproite pipes.
'Blood' diamonds
In some of the more politically unstable central African and west African countries, revolutionary groups have taken control of diamond mines, using proceeds from diamond sales to finance their operations. Diamonds sold through this process are known as conflict diamonds or blood diamonds. In response to public concerns that their diamond purchases were contributing to war and human rights abuses in central Africa and West Africa, the United Nations, the diamond industry and diamond-trading nations introduced the Kimberley Process in 2002, which is aimed at ensuring that conflict diamonds do not become intermixed with the diamonds not controlled by such rebel groups. The Kimberley Process provides documentation and certification of diamond exports from producing countries to ensure that the proceeds of sale are not being used to fund criminal or revolutionary activities. Although the Kimberley Process has been moderately successful in limiting the number of conflict diamonds entering the market, conflict diamonds smuggled to market continue to persist to some degree. Two major flaws still hinder the effectiveness of the Kimberley Process: the relative ease of smuggling diamonds across African borders and giving phony histories, and the violent nature of diamond mining in nations which are not in a technical state of war and whose diamonds are therefore considered "clean."[26]
The Canadian Government has setup a body known as Canadian Diamond Code of Conduct to help authenticate Canadian Diamonds. This is a very stringent tracking system of diamonds and helps protect the 'conflict free' label of Canadian diamonds.
Currently, gem production totals nearly 30 million carats (6,000 kg) of cut and polished stones annually, and over 100 million carats (20,000 kg) of mined diamonds are sold for industrial use each year, as are about 100,000 kg of synthesized diamond. In 2003, this constituted total production of nearly US$9 billion in value.
Mining
Only a very small fraction of the diamond ore consists of actual diamonds. The ore is crushed, during which care has to be taken in order to prevent larger diamonds from being destroyed in this process and subsequently the particles are sorted by density. Today, diamonds are located in the diamond-rich density fraction with the help of X-ray fluorescence, after which the final sorting steps are done by hand. Before the use of X-rays became commonplace, the separation was done with grease belts; diamonds have a stronger tendency to stick to grease than the other minerals in the ore.
Distribution
The Diamond Trading Company, or DTC, is a subsidiary of De Beers and markets rough diamonds produced both by De Beers mines and other mines from which it purchases rough diamond production. DTC performs sophisticated sorting of rough diamonds into categories, and then sells bulk lots of rough diamonds to a limited number of sightholders a few times a year.
Once purchased by sightholders, diamonds are cut and polished in preparation for sale as gemstones. The cutting and polishing of rough diamonds is a specialized skill that is concentrated in a limited number of locations worldwide. Traditional diamond cutting centers are Antwerp, Amsterdam, Johannesburg, New York, and Tel Aviv. Traditionally, diamond cutters in these cities are Orthodox Jews or Chasidim. Recently, diamond cutting centers have been established in China, India, and Thailand. Cutting centers with lower costs of labor, notably Surat in Gujarat, India, handle a larger number of smaller carat diamonds, while smaller quantities of larger or more valuable diamonds are more likely to be handled in Europe or North America. The recent expansion of this industry in India, employing low cost labor, has allowed smaller diamonds to be prepared as gems than was previously economically feasible.
Diamonds which have been prepared as gemstones are sold on diamond exchanges called bourses. This is the final tightly controlled step in the diamond supply chain; wholesalers and even retailers are able to buy relatively small lots of diamonds at the bourses, after which they are prepared for final sale to the consumer. Diamonds can be sold already set in jewelry, or as is increasingly popular, sold unset ("loose").
Synthetics, simulants, and enhancements
Natural diamonds have formed naturally within the earth. Synthetic diamonds are created by a man-made process. A diamond simulant is defined as a non-diamond material that is used to simulate the appearance of a diamond.
The gemological and industrial uses of diamond have created a large demand for rough stones. The demand for industrial diamonds has long been satisfied in large part by synthetic diamonds, which have been manufactured by various processes for more than half a century. However, in recent years it has become possible to produce gem-quality synthetic diamonds of significant size.[5]
The majority of commercially available synthetic diamonds are yellow in color and produced by so called High Pressure High Temperature (HPHT) processes.[27] The yellow color is caused by Nitrogen impurities. Other colors may also be reproduced such as blue, green or pink which are a result of the addition of Boron or from irradiation after synthetisation.[28]
At present the annual production of gem quality synthetic diamonds is only a few thousand carats, whereas the total production of natural diamonds is around 120 million carats. Although the production of colorless synthetic diamonds is dwarfed by that of natural diamonds, one can only find one fancy colored diamond for every 10.000 colorless ones. Since almost the complete production of synthetic diamonds consists of fancy diamonds, there is a high probability that the larger fancy colored diamonds (over 1.5 carats) will be synthetic.[29]
Currently, trained gemologists can also distinguish between natural diamonds from synthetic diamonds. Although it has been claimed that synthetic diamonds are so perfect that it is virtually impossible to distinguish them from natural diamonds, this is not the case. Depending on the type of diamonds (either HPHT produced or CVD produced) and the color of the diamond (colored, D-Z color range or D-J color range) several methods of identification are at the disposal of a gemologist or gemlab: CVD diamonds can be identified through their orange fluorescence, D-J colored diamonds can be screened through the Swiss Gemological Organization's (SSEF)[30] Diamond Spotter and stones in the D-Z color range can be identified through the DiamondSure UV/visible spectrometer which is a tool developed by De Beers.[31]
A diamond's gem quality, which is not as dependent on material properties as industrial applications, has invited both imitation and the invention of procedures to enhance the gemological properties of natural diamonds. Materials which have similar gemological characteristics to diamond but are not mined or synthetic diamond are known as diamond simulants. The most familiar diamond simulant to most consumers is cubic zirconia (commonly abbreviated as CZ); recently moissanite has also gained popularity and has often been mischaracterized as a diamond simulant, although it is sold and retailed as a replacement for diamond. Both CZ and moissanite are synthetically produced. However, CZ is a diamond simulant. Diamond enhancements are specific treatments, performed on natural diamonds (usually those already cut and polished into a gem), which are designed to better the gemological characteristics of the stone in one or more ways. These include laser drilling to remove inclusions, application of sealants to fill cracks, treatments to improve a white diamond's color grade, and treatments to give fancy color to a white diamond.
Currently, trained gemologists with appropriate equipment are able to distinguish natural diamonds from simulant diamonds, and they can identify all enhanced natural diamonds. Coatings are more and more used to give a diamond simulant such as Cubic Zirconia a more "Diamond like" appearance. One such substance, which is heavily advertised, is what scientists refer to as "diamond-like carbon." This is an amorphous carbonaceous material that has some physical properties which are similar to that of the diamond. Advertising suggests (righfully so or not) that such a coating would transfer some of these diamond-like properties to the coated stone, hence enhancing the diamond simulant. However modern techniques such as Raman Spectroscopy should easily identify such as treatment.[32]
Producing large synthetic diamonds threatens the business model of the diamond industry, and the ultimate effect of the ready availability of gem-quality diamonds at low cost in the future is hard to predict at this time.
Symbolism and Lore
Historically, it has been claimed that diamonds possess several supernatural powers:
- A diamond gives victory to him who carries it bound on his left arm, no matter the number of enemies.[33]
- Panics, pestilences, enchantments, all fly before it; hence, it is good for sleepwalkers and the insane.[33]
- It deprives lodestone and magnets of their virtue (i.e., ability to attract iron).[33]
- Arabic diamonds are said to attract iron greater than a magnet.[33]
- A diamond's hardiness can only be broken by smearing it with fresh goat's blood.[33]
- In traditional Hinduism one should avoid contact with a diamond which surface area is damaged by a crack, a crowfoot, round, dull, speckled area or which is black-blue, flat, and if uncut, other than the (ideal) hexagonal shape.[34]
Because of their extraordinary physical properties, diamonds have been used symbolically since near the time of their first discovery. Perhaps the earliest symbolic use of diamonds was as the eyes of Hindu devotional statues.[35] In Hinduism Indra uses Vajrayudham or the thunderbolt as his primary weapon. Vajra is the word for diamond and ayudham means weapon in Sanskrit. Another name for it was Agira which means fire or the sun. In fact there are 14 names counted to be given to a diamond in traditional Hinduism.[34]
The oldest dated printed book in the world is called the Diamond Sutra, dates from 868 C.E. and was found in a cave in North-West China. Sutra's are most used to describe the teachings of Buddha. In this case the title of the Sutra refers not to the diamond itself but to a 'diamond blade that will cut through worldly illusion to illuminate what is real and everlasting'. Jewel imaginary forms a central part of Buddhism: the triple-jewel represent 'Buddha', his teachings Dharma and the spiritual community Shangha. The book presently resides in the British Library.[36]
Many cultures use divine intervention to explain the origin and creation of gemstones, and diamonds were no exception to this. In Greek mythology for example it was the youth on the island of Crete that disturbed Zeus and who were then (as a form of punishment) transformed into the adamas.[37]
Philosophers however had a more naturalistic approach to explain the origin of gems: Plato for example believed gemstones were a consequence of fermentation in the stars, where a diamond actually formed the kernel of gold-bearing mass.[37] In fact often diamonds were linked to gold, which may have found its origin in the joint occurrence of diamonds with quartzite, quartz veins and an occasional occurrence of gold in them.[38]
In later times, Robert Boyle actually believed that gems (including a diamond) were formed of clear, transparent water, and that their colors and characteristics were derived from their metallic spirit.[39]
The Ring
The origin of our custom to use diamonds in rings, and more recently, in engagement rings, can be traced back to the Middle Ages and even the Romans. The Romans valued the diamond entirely on account of its supernatural powers. Pliny wrote that a diamond baffles poison, keeps off insanity and dispels vain fears.[38] The medieval Italians copied these beliefs and added some to it: they called it the "Pietra della Reconciliazone" because it maintained concord between husband and wife. On this account it was recommended as the stone to be set in wedding (or espousal) rings—not on account of its beauty therefore, which was described by Isidore of Seville as a small stone devoid of beauty.[38]
In more recent times a Parisian Oracle of mystic subjects, the Baron d'Orchamps, announced the diamond, if worn on the left (hand) warded off evil influences and attracted good fortune and since he had fashionable clients the word spread and the wearing of the diamond on the left hand became in itself a fashion.[40]
One of the first occurrences of the diamond engagement (or wedding) ring can be traced back to the marriage of Maximilian I (then Archduke of Austria) to Mary of Burgundy in 1477.[41] Other early examples of betrothal jewels incorporating diamonds include the Bridal Crown of Blanche (ca. 1370–80)[41] and the Heftlein brooch of Vienna (ca. 1430–40),[41] a pictorial piece depicting a wedding couple.
The popularity of the diamond ring as an engagement ring for a much wider audience can be traced directly to the marketing campaigns of De Beers, starting in 1938.[42] Such a campaign had become necessary to sell the large quantity of diamonds suddenly available because of the large diamond finds particularly in South Africa.
Other Facts
The diamond is the birthstone for people born in the month of April, and is also used as the symbol of a 60-year anniversary, such as a Diamond Jubilee (see hierarchy of precious substances). In a system of heraldry by gemstones occasionally used in the past for the arms of nobles, diamond was used to represent the color sable, or black.[43]
Diamonds are a common focus of fiction. Notable pieces of fiction include Ian Fleming's Diamonds Are Forever (1956), Arthur C. Clarke's 2061: Odyssey Three (1988), F. Scott Fitzgerald's "The Diamond as Big As the Ritz" (1922), and Neal Stephenson's The Diamond Age (1995). In addition, diamonds are the subject of various myths and legends.
See also
Notes
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Gemological Institute of America, GIA Gem Reference Guide (Carlsbad, CA: Gemological Institute of America, 1995, ISBN 0873110196).
- ↑ Pliny the Elder, Natural History: A Selection (New York, NY: Penguin Classics, 1991, ISBN 0140444130).
- ↑ 3.0 3.1 "Chinese made first use of diamond," BBC News (May 17, 2005). Retrieved June 21, 2019.
- ↑ 4.0 4.1 4.2 4.3 Gemological Institute of America, Diamonds and Diamond Grading: Lesson 4 How Diamonds Form Carlsbad, CA: Gemological Institute of America, 2002.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 George E. Harlow (ed.), The Nature of Diamonds (Cambridge University Press, 1997, ISBN 0521629357).
- ↑ M. Sevdermish and A. Mashiah, The Dealer's Book of Gems and Diamonds (Israel: Kal Printing House, 1995).
- ↑ Robert Webster and Peter G. Read (eds.),Gems: Their sources, descriptions and identification (Burlington, MA: Butterworth-Heinemann, 2000, ISBN 0750616741).
- ↑ Yinon Bentor, Carbon Melting and Boiling Points ChemicalElements.com. Retrieved June 21, 2019.
- ↑ What are Aggregated Diamond Nanorods?. WiseGeek. Retrieved July 2, 2019.
- ↑ W.R. Taylor, A.J. Lynton, and M. Ridd, Nitrogen defect aggregation of some Australasian diamonds: Time-temperature constraints on the source regions of pipe and alluvial diamonds. American Mineralogist. 75 (1990):1290–1310. Retrieved July 2, 2019.
- ↑ J.E. Field, Strength and Fracture Properties of Diamond. Philosophical Magazine A 43(3) (1981):595–618.
- ↑ Why Turn to GIA for Gem Grading and Analysis?. GIA. Retrieved July 2, 2019.
- ↑ Antwerp World Diamond Center Retrieved July 2, 2019.
- ↑ Introduction to the Rapaport Price List. Retrieved July 2, 2019.
- ↑ ASET® Images of Diamonds American Gem Society. Retrieved July 2, 2019.
- ↑ A Contribution to the Understanding of Blue Fluorescence on the Appearance of Diamonds. GIA. Retrieved July 2, 2019.
- ↑ 17.0 17.1 17.2 J. Willard Hershey, The Book of Diamonds (New York, NY: Hearthside Press, 1940).
- ↑ Earliest use of diamonds by Chinese found. China Daily. Retrieved July 2, 2019.
- ↑ In fact Pliny in Book XXXVII, xv, 61 mentioned Germany as the best location of Diamonds. E. Caley and J. Richards also discuss Pliny's referral in Book XXXVI, 54 of the stone of "Naxos" as being adamas, and which had long been used for cutting and polishing. A chief product of Naxos has long been a high grade of amorphous corundum which was used as an abrasive. ("Theophrastus, On Stones," E. Caley, J. Richards, (Ohio State University, 1956), 91). They further discuss his referral to the adamas coming from the "East" through Armenian traders but they show this was actually based on an erroneous interpretation of Theophrastus. Gardner F. Williams, Diamond Mines of South Africa (New York: BF Buck Company, 1905) argues that the stone named "adamas" by the Greek and further referred to by Pliny was most likely a sapphire since this was a much more abundant stone, even amongst traders in Asia, than diamond (especially when used in the context of "adamas" being an ornamental stone and not used in an "industrial" context of engraving gems). Streeter makes a similar argument in his book (Edwin Streeter, Precious Stones and Gems (London: Bell and Sons, 1898). There seems to be a consensus over a large period of time that in fact the "adamas" was not a diamond, but mostly any type of Corundum, several other minerals such as Spinel were probably confused with diamonds as well; particularly because of a similarity in hardness and their availability in the Mediterranean area.
- ↑ David Whitehouse, Diamond star thrills astronomers. BBC, February 16, 2004. Retrieved July 2, 2019.
- ↑ Peter Greste, BBC NEWS, 'Massive' gem dug up in S Africa. BBC, August 28, 2007. Retrieved July 2, 2019.
- ↑ Fresh tests for 'giant diamond'. BBC, August 30, 2007. Retrieved July 2, 2019.
- ↑ W.R. Catelle, The Diamond (London; New York: John Lane Company, 1911). Discussion on Alluvial diamonds in India and elsewhere as well as earliest finds.
- ↑ V. Ball, Diamonds, Gold and Coal of India (London, UK: Truebner & Co., 1881). Ball was a Geologist in British service.
- ↑ 25.0 25.1 V. Lorenz, Argyle in Western Australia: The world's richest diamondiferous pipe; its past and future. Gemmologie, Zeitschrift der Deutschen Gemmologischen Gesellschaft 56(1/2) (2007):35-40.
- ↑ Tom Zoellner, The Heartless Stone: A Journey Through the World of Diamonds, Deceit, and Desire (New York, NY: St. Martin's Press, 2006, ISBN 0312339690).
- ↑ J.E. Shigley, et al., Gemesis Laboratory Created Diamonds. Gems and Gemology 38(4) (2002):301–309.
- ↑ J.E. Shigley et al., Lab Grown Colored Diamonds from Chatham Created Gems. Gems and Gemology 40(2) (2004):128-145.
- ↑ Michael O'Donoghue Gems (St. Louis, MO: Elsevier, 2006, ISBN 0750658568).
- ↑ SSEF Swiss Gemmological Institute. Retrieved July 3, 2019.
- ↑ Christopher Welbourn, Identification of Synthetic Diamonds: Present Status and Future Developments/Proceedings of the 4th International Gemological Symposium. Gems and Gemology 42(3) (2006):34-35.
- ↑ J.E. Shigley, Observations on new coated gemstones. Gemmologie: Zeitschrift der Deutschen Gemmologischen Gesellschaft 56(1/2) (2007):53-56.
- ↑ 33.0 33.1 33.2 33.3 33.4 Lewis Spence, An Encyclopaedia of Occultism (University Books, Inc., 1960).
- ↑ 34.0 34.1 Richard Garbe, Die Indischen Mineralien, Ihre Nahmen und Ihre Zugeschriebene Kraefte. (Leipzig, Germany: Verlag von S. Hirzel, 1882).
- ↑ W.R. Catelle, The Diamond. (John Lane Company, 1911). He describes Diamonds being worn as long as 5000 years ago, recorded in ancient Hindu writings. George Frederick Kunz. in "A Curious Lore of Precious Stones" describes how a diamond in ancient times is worn on the forehead until the early nineteenth century this was a tradition believed to bring good luck. Devotional statues are not specifically mentioned.
- ↑ Diamond Sutra. British Library. Retrieved July 3, 2019.
- ↑ 37.0 37.1 S.M. Burnham, Precious Stones in Nature, Art and Literature (Boston, MA: Bradlee Whidden, 1886).
- ↑ 38.0 38.1 38.2 C.W. King A Natural History of Precious Stones and Precious Metals (Cambridge Press, 1897).
- ↑ Robert Boyle, An Essay about the Origin and Virtue of Gems. (London, UK: William Godbid, 1672).
- ↑ James Remington McCarthy Fire in the Earth, the Story of the Diamond (New York, NY: Harper and Brothers, 1942).
- ↑ 41.0 41.1 41.2 George Frederick Kunz, Rings for the Finger (Philadelphia, PA: J.H. Lippincott Co., 1917).
- ↑ E. J. Epstein, Have you ever tried to sell a diamond? The Atlantic, February, 1982. Retrieved July 3, 2019.
- ↑ Julian Franklyn, Shield and Crest (London, UK: MacGibbon & Kee, 1961).
ReferencesISBN links support NWE through referral fees
- Catelle, W.R. The Diamond. London; New York: John Lane Company, 1911.
- David, Joshua. "The New Diamond Age". Wired, 2003. Retrieved July 9, 2020.
- Epstein, Edward Jay. "Have You Ever Tried To Sell a Diamond?" The Atlantic Monthly, 1982. Retrieved July 9, 2020.
- Epstein, Edward Jay. The Diamond Invention. Lancashire, UK: Arrow Publishing, 1982. ISBN 0091476909
- Eppler, W.F. Praktische Gemmologie. Rühle-Diebner-Verlag, 1989.
- Evevn-Zohar, Chaim. "From Mine to Mistress - Corporate Strategies and Government Policies in the International Diamond Industry" (Second edition of the book on the world diamond industry) Mining Journal Press, 2007.
- Franklyn, Julian. Shield and Crest. London, UK: MacGibbon & Kee, 1961.
- Gemological Institute of America. GIA Gem Reference Guide. Carlsbad, CA: Gemological Institute of America, 1995. ISBN 0873110196
- Harlow, George E., (ed.). The Nature of Diamonds. Cambridge University Press, 1997. ISBN 0521629357
- Hershey, J. Willard. The Book of Diamonds: Their Curious Lore, Properties, Tests and Synthetic Manufacture 1940. Kessinger Publishing, LLC., 2010. ISBN 1162734892
- King, C.W. A Natural History of Precious Stones and Precious Metals. Cambridge Press, 1897.
- Kjarsgaard, B.A., and A.A. Levinson. Diamonds in Canada. Gems & Gemology 38(3) (2002): 208-238.
- Kunz, George Frederick. Curious Lore of Precious Stones. Philadelphia, PA: Lippincott Co., 1913.
- Kunz, George Frederick. Rings for the Finger. Philadelphia, PA: J.H. Lippincott Co., 1917.
- O'Donoghue, Michael. Gems. St. Louis, MO: Elsevier, 2006. ISBN 0750658568
- Pagel-Theisen, Verena. Diamond Grading ABC: the Manual. Antwerp, Belgium: Rubin & Son, 2001. ISBN 3980043460
- Streeter. The Great Diamonds of the World. London, UK: George Bell & Sons, 1882.
- Taylor, W.R., A.J. Lynton & M. Ridd. Nitrogen defect aggregation of some Australasian diamonds: Time-temperature constraints on the source regions of pipe and alluvial diamonds. American Mineralogist 75 (1990):1290–1310. Retrieved July 9, 2020.
- Tolkowsky, Marcel. Diamond Design: A Study of the Reflection and Refraction of Light in a Diamond. London, UK: E. & F.N. Spon, Ltd., 1919. Retrieved July 9, 2020.
- Tyson, Peter. "Diamonds in the Sky". Nova PBS.org, 2000. Retrieved July 9, 2020.
- Webster, Robert, and Peter G. Read, (eds.). Gems: Their sources, descriptions and identification. Burlington, MA: Butterworth-Heinemann, 2000. ISBN 0750616741
- Weiner, K.L., R. Hochleitner, S. Weiss, H. Voelstadt. Diamant. München: Lapis, 1994.
- Welbourn, Christopher. Identification of Synthetic Diamonds: Present Status and Future Developments. (Proceedings of the 4th International Gemological Symposium.) Gems and Gemology 42(3) (2006): 34-35.
- Williams, Gardner. The Diamond Mines of South Africa. New York, NY: B.F Buck & Co., 1905.
- Wise, Richard W. Secrets Of The Gem Trade, The Connoisseur's Guide To Precious Gemstones. Lenox, MA: Brunswick House Press, 2003. ISBN 0972822380
- Yarnell, Amanda. "The Many Facets of Man-Made Diamonds". Chemical & Engineering News 82(5) (2004): 26-31. Retrieved July 9, 2020.
- Zoellner, Tom. The Heartless Stone: A Journey Through the World of Diamonds, Deceit, and Desire. New York, NY: St. Martin's Press, 2006. ISBN 0312339690
External links
All links retrieved July 28, 2022.
| Common jewelry materials | |
|---|---|
| Precious metals: | Gold · Silver (Sterling silver) · Platinum · Palladium |
| Gemstones: | Diamonds · Rubies · Emeralds · Sapphires · Amethyst · Opals · Topaz · Aquamarine · Tanzanite · Alexandrite |
| Others: | Amber · Pearls |
| Gemstones and crystals | |
|---|---|
| Gems: | Aquamarine · Emerald · Jasper · Lapis lazuli · Pearl · Peridot · Ruby · Sunstone · Tiger's eye |
| Crystals: | Agate · Amethyst · Chalcedony · Diamond · Pyrite · Quartz · Rhodochrosite · Sapphire · Topaz · Tourmaline |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.