Materials science is an interdisciplinary field involving the study of different types of materials and the applications of knowledge about these materials to various areas of science and engineering. It combines elements of applied physics and chemistry, as well as chemical, mechanical, civil and electrical engineering. Materials science and materials engineering are often combined into a larger field of study.
Materials used in early human history included metals, glasses, and clay-based ceramics. The past century has witnessed a surge in the development of new materials, including plastics, advanced ceramics, semiconductors, superconductors, liquid crystals, Bose-Einstein condensates, and nanoscale substances, with a wide range of applications. Furthermore, materials science has grown to include testing these more exotic forms of condensed matter and developing new physics theories to explain their behavior. Consequently, materials science has been propelled to the forefront at many academic institutions and research facilities.
Materials research at the basic level can lead to unprecedented influence on society. For example, semiconductor materials, which are ubiquitous in cars, telephones, computers, clocks, kitchen appliances, children’s toys, satellites, telescopes, and more, were a product of materials science research—into the electronic properties of the element germanium. Further research led to the replacement of germanium with the less costly silicon and to diverse approaches to modifying silicon’s properties by implanting other elements, such as phosphorous or boron, into the silicon matrix. Since their discovery in 1947, semiconductors have been steadily improved through materials science research driven by ever-increasing performance demands from the computer industry.
Efforts to apply ethical considerations to Materials Science quickly reach what is a common barrier between ethics and the combined fields of science and technology. An individual scientist, for example, who would want to conduct research toward such a noble goal as developing a light-weight and durable structural plastic that is readily recyclable must first either find and join a research group that is already funded to support such research or find an independent funding source for such research.
Historical overview
Materials science is one of the oldest forms of applied science and engineering. In the history of human civilization, different eras have often been retrospectively identified according to an advance in the human ability to work with a new type of material. Examples are the Stone Age, Bronze Age, and Iron Age. A major breakthrough in the understanding of materials occurred in the late nineteenth century, when Willard Gibbs demonstrated that thermodynamic properties relating to atomic structure in various phases are related to the physical properties of a material.
Before the 1960s, (and in some cases decades after), many materials science departments at academic and research institutions were named metallurgy departments, because the emphasis was on the study of metals and their uses. The field has since broadened to include every class of materials, such as ceramics, polymers, semiconductors, superconductors, superfluids, magnetic materials, medical implant materials, and biological materials.
Many important elements of modern materials science have resulted from the space race. In particular, the understanding and engineering of metallic alloys, ceramics, and other materials were useful for the construction of space vehicles, space suits, and so forth, and the new knowledge was found valuable for various consumer and industrial applications as well. Materials science has laid the physical foundations of 21st century civilization, being integral to everything from fiber optic cables to tennis shoes, and from solar cells to sail boats. Materials science will continue to be centrally important in the quest for finding technological solutions toward sustainable development in the face of environmental degradation and the continued buildup of greenhouse gases due to the burning of carbon-based fuels.
Fundamentals of materials science
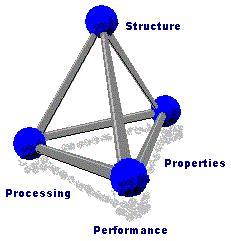
In materials science, the researcher conducts a systematic investigation of each material, in terms of its structure, properties, processing, and performance. The research often leads to new applications of known materials and the creation of new materials with desired properties.
On a fundamental level, this field relates the properties and performance of a material to its atomic-scale structure and the different phases it can go through. The major factors that determine the structure and properties of a material are the nature of its constituent chemical elements and the way in which the material was processed into its final form. These factors, related through the laws of thermodynamics, govern the material’s microstructure, and thus its properties.
An old adage in materials science says: "materials are like people; it is the defects that make them interesting". Given the limits of today's technology, that is good, because the manufacture of a perfect crystal of a material is physically impossible. Instead, materials scientists manipulate a material's defects to create materials with the desired properties. On an atomic scale, the defects in a crystal could mean that atoms of one element may be missing or replaced by atoms of other elements.
Not all materials have a regular crystalline structure. Glasses and some ceramics—unlike many natural materials— are amorphous, that is, they do not possess any long-range order in their atomic arrangements. Engineering these materials is much more difficult than engineering crystalline materials. Polymers may exhibit varying degrees of crystallinity, and studying them requires a combination of elements of chemical and statistical thermodynamics to give thermodynamic (rather than mechanical) descriptions of physical properties.
Materials in Industry
Radical advances in understanding and manipulating materials drive the creation of new products and even new industries. At the same time, stable industries employ materials scientists to make incremental improvements and troubleshoot issues with currently used materials. Industrial applications of materials science include the design of materials and their cost-benefit tradeoffs in industrial production.
Techniques used for processing materials include:
Techniques used for analyzing (characterizing) materials include:
- electron microscopy
- X-ray diffraction
- calorimetry
- nuclear microscopy (HEFIB)
- Rutherford backscattering
- neutron diffraction
The overlap between physics and materials science has lent itself naturally the development of the interface field of materials physics, which is concerned with the physical properties of materials. The approach is generally more macroscopic and applied than in condensed matter physics.
Classes of materials
Materials science encompasses various classes of materials, some of which overlap. Examples are:
- Ionic crystals (crystals in which the atoms are held together by ionic bonds)
- Covalent crystals (crystals in which the atoms are held together by covalent bonds)
- Vitreous (glassy) materials
- Metals
- Intermetallics
- Polymers
- Composite materials
- Biomaterials (materials derived from or intended for use with biological systems)
- Electronic and magnetic materials (materials such as semiconductors used to create integrated circuits, storage media, sensors, and other devices)
- Ceramics and refractories (high-temperature materials, including reinforced carbon-carbon (RCC), polycrystalline silicon carbide, and transformation-toughened ceramics)
Each class of materials may involve a separate field of study.
Subfields of materials science
- Nanotechnology: As commonly understood, nanotechnology is the field of applied science and technology concerned with the formation, study, and control of materials having a width ranging from less than 1 nanometer (10−9 meter) to 100 nanometers. These materials are generally engineered on a molecular scale. On a more rigorous level, nanoscience involves the study of materials whose defining properties are present only at the nanoscale.
- Crystallography: This is the study of the arrangement of atoms in a crystalline solid and the relationship between the crystalline structures and their physical properties. It includes the determination of defects associated with crystal structures.
- Materials characterization: Information needed for understanding and defining the properties of materials is acquired through such techniques as diffraction of X-rays, electrons, or neutrons, and various forms of spectroscopy, chromatography, thermal analysis, or electron microscopy.
- Metallurgy: This involves the study of metals and their alloys, including their extraction, microstructure, and processing.
- Tribology: This is the study of the wear of materials due to friction and other factors.
- Surface science: It involves study of the structures and interactions occurring at the interfaces of solids and gases, solids and liquids, and solids and solids.
- Glass science: It involves the study of noncrystalline materials, including inorganic glasses, vitreous metals, and non-oxide glasses.
Some practitioners consider rheology a subfield of materials science, because it can cover any material that flows. Modern rheology, however, typically deals with non-Newtonian fluid dynamics, so it is often considered a subfield of continuum mechanics.
Topics that form the basis of materials science
- Thermodynamics, statistical mechanics, chemical kinetics, and physical chemistry: to understand phase stability and physical and chemical transformations.
- Chemical bonding: to understand the bonds between atoms of the material.
- Mechanics of materials: to understand the mechanical properties of materials and their structural applications.
- Solid-state physics and quantum mechanics: to understand the electronic, thermal, magnetic, chemical, structural, and optical properties of materials.
- Solid-state chemistry and polymer science: to understand the properties of polymers (including plastics), colloids, ceramics, and liquid crystals.
- Biology: for the integration of materials into biological systems.
- Continuum mechanics and statistics: for the study of fluid flows and ensemble systems.
- Diffraction and wave mechanics: for the characterization of materials.
Timeline of materials technology
Before Common Era
- 29,000–25,000 B.C.E. - First ceramic appears
- Third millennium B.C.E. - Copper metallurgy is developed and copper is used for ornamentation
- Second millennium B.C.E. - Bronze is used for weapons and armor
- Sixteenth century B.C.E. - The Hittites develop crude iron metallurgy
- Thirteenth century B.C.E. - Invention of steel, when iron and charcoal are appropriately combined
- First millennium B.C.E. - Pewter begins to be used in China and Egypt
- Tenth century B.C.E. - Glass production begins in Greece and Syria
- 50s B.C.E. - Glassblowing techniques flourish in Phoenicia
- 20s B.C.E. - Roman architect Vitruvius describes low-water-content method for mixing concrete
First millennium
- 700s - Porcelain is invented in China
Second millennium
- 1448 - Johannes Gutenberg develops type metal alloy
- 1450s - Cristallo, a clear soda-based glass is invented by Angelo Barovier
- 1590 - Glass lenses are developed in the Netherlands and used for the first time in microscopes and telescopes
Eighteenth century
- 1738 - William Champion patents a process for the production of metallic zinc by distillation from calamine and charcoal
- 1740 - Benjamin Huntsman developed the crucible steel technique
- 1779 - Bry Higgins issued a patent for hydraulic cement (stucco) for use as an exterior plaster
- 1799 - Alessandro Volta makes a copper/zinc acid battery
Nineteenth century
- 1821 - Thomas Johann Seebeck invents the thermocouple
- 1824 - Patent issued to Joseph Aspin for portland cement
- 1825 - Hans Christian Ørsted produces metallic aluminum
- 1839 - Charles Goodyear invents vulcanized rubber
- 1839 - Louis Daguerre and William Fox Talbot invent silver-based photographic processes
- 1855 - Bessemer process for mass production of steel patented
- 1861 - James Clerk Maxwell demonstrates color photography
- 1883 - Charles Fritts makes the first solar cells using selenium wafers
Twentieth century
- 1902 - Auguste Verneuil develops the Verneuil process for making synthetic rubies
- 1909 - Leo Baekeland presents Bakelite, a hard, thermosetting plastic
- 1911 - Heike Kamerlingh Onnes discovers superconductivity
- 1912 - Harry Brearley invents stainless steel
- 1916 - Jan Czochralski invents a method for growing single crystals of metals
- 1924 - Corning Glass Works scientists invent Pyrex, a glass with a very low coefficient of thermal expansion
- 1931 - Julius Nieuwland develops the synthetic rubber called neoprene
- 1931 - Wallace Carothers develops nylon
- 1938 - Roy Plunkett discovers the process for making poly-tetrafluoroethylene, better known as teflon
- 1947 - First germanium transistor invented
- 1947 - First commercial application of a piezoelectric ceramic: barium titanate used as a phonograph needle
- 1951 - Individual atoms seen for the first time, using the field ion microscope
- 1953 - Karl Ziegler discovers metallic catalysts, allowing the production of polyethylene polymers with greatly improved strength
- 1954 - Six percent efficiency silicon solar cells made at Bell Laboratories
- 1959 - Pilkington Brothers patent the float glass process
- 1962 - Invention of SQUID (superconducting quantum interference device)
- 1968 - Liquid crystal display (LCD) developed by RCA
- 1970 - Silica optical fibers grown by Corning Incorporated
- 1970 - Invention of AOD (argon oxygen decarburization) refining
- 1980 - Development of duplex stainless steels that resist oxidation in chlorides
See also
ReferencesISBN links support NWE through referral fees
- Askeland, Donald R. and Pradeep P. Phulé (2005). The Science & Engineering of Materials, 5th edition, Thomson-Engineering. ISBN 0534553966.
- Eberhart, Mark (2003). Why Things Break: Understanding the World by the Way It Comes Apart. Harmony. ISBN 1400047609.
- Gaskell, David R. (1995). Introduction to the Thermodynamics of Materials, 4th edition, Taylor and Francis Publishing. ISBN 1560329920.
- Gordon, James Edward (1984). The New Science of Strong Materials or Why You Don't Fall Through the Floor, Reissue edition, Princeton University Press. ISBN 0691023808.
External links
All links retrieved November 7, 2022.
| ||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.