Nucleoside
Nucleosides are structural subunits of nucleic acids, the macromolecules that convey genetic information in living cells. They consist of a nitrogen-containing base bonded to a five-carbon (pentose) sugar.
Nucleosides are the biochemical precursors of nucleotides, the molecular building blocks of the nucleic acids DNA and RNA. Nucleotides also are important in cell metabolism (ATP is the energy currency of the cell) and as co-enzymes. Nucleotides are formed by the addition of one or more phosphate groups to the nucleoside.
Some nucleosides have important clinical applications; for example, puromycin and certain other antibiotics are nucleosides produced by molds or fungi.
Human creativity is also shown in the ability of drug researchers to draw on an understanding of the biochemistry of naturally occurring nucleosides to build synthetic molecules called nucleoside analogs. One class of antiretroviral drugs is called nucleoside analog reverse transcriptase inhibitors (NARTIs or NRTIs). NRTIs inhibit the activity of reverse transcriptase, a viral DNA polymerase enzyme needed by HIV in order to reproduce. When HIV infects a cell, reverse transcriptase copies the single-stranded RNA genome of the virus into a double-stranded viral DNA molecule. The viral DNA is then integrated into the host’s chromosomal DNA, which allows the host to reproduce the virus. NRTIs block reverse transcriptase's enzymatic function, disrupting the synthesis of the double-stranded viral DNA and thus preventing HIV from multiplying. In order to be incorporated into the viral DNA, NRTIs must be activated in the cell by the addition of three phosphate groups to form NRTI triphosphates.
The chemical components of nucleosides
The nitrogen-containing base of a nucleoside (also called the nucleobase) is typically a derivative of either purine or pyrimidine, which are heterocyclic compounds (organic compounds that contain a ring structure that has, in addition to carbon, such atoms as sulfur, oxygen, or nitrogen). The most common bases in nucleosides are:
The sugar component is either deoxyribose or ribose. (“Deoxy” simply indicates that the sugar lacks an oxygen atom present in ribose, the parent compound.)
Below is a table listing the common bases and their corresponding nucleosides:
| Nitrogenous base | Nucleoside | Deoxynucleoside |
|---|---|---|
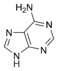 Adenine |
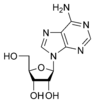 Adenosine A |
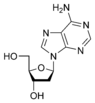 Deoxyadenosine dA |
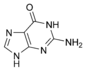 Guanine |
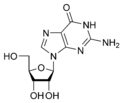 Guanosine G |
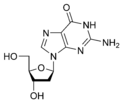 Deoxyguanosine dG |
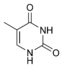 Thymine |
 5-Methyluridine m5U |
 Deoxythymidine dT |
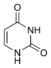 Uracil |
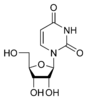 Uridine U |
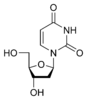 Deoxyuridine dU |
 Cytosine |
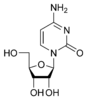 Cytidine C |
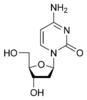 Deoxycytidine dC |
Nucleosides are nucleotide precursors
A nucleotide is a phosphate ester of a nucleoside. In chemistry, esters are organic compounds in which an organic group replaces a hydrogen atom or multiple hydrogens in an oxygen acid. Here, the hydroxyl group of the nucleoside, attached to carbon atom 5 of the sugar unit, is replaced by one or more phosphate groups.
Nucleotides are named according to the nucleoside that corresponds to the base. For example, the nucleotide adenosine triphosphate (ATP) is a derivative of the nucleoside adenosine.
The breakdown of nucleosides
There is a continuous turnover of nucleotides in the cell. Nucleosides are derived in the second step of nucleic acid degradation when a class of enzymes called nucleotidases split nucleotides into their component nucleosides and phosphate groups. The nucleosides, in turn, are subsequently broken down:
- In the lumen of the digestive system by nucleosidases into nitrogenous bases and ribose (or deoxyribose).
- Inside the cell by nucleoside phosphorylases into nitrogenous bases and ribose-1-phosphate (or deoxyribose-1-phosphate).
ReferencesISBN links support NWE through referral fees
- Lindahl, T. 1993. "Instability and Decay of the Primary Structure of DNA." Nature 362(6422): 709–715.
- Stryer, L. 1988. Biochemistry, 4th edition. New York, NY: W. H. Freeman. ISBN 071671843X.
- Watson, J. D., and Crick, F. H. C. 1953. A structure for deoxyribose nucleic acid (PDF). Nature 171: 737–738.
| Nucleic acids edit |
|---|
| Nucleobases: Adenine - Thymine - Uracil - Guanine - Cytosine - Purine - Pyrimidine |
| Nucleosides: Adenosine - Uridine - Guanosine - Cytidine - Deoxyadenosine - Thymidine - Deoxyguanosine - Deoxycytidine |
| Nucleotides: AMP - UMP - GMP - CMP - ADP - UDP - GDP - CDP - ATP - UTP - GTP - CTP - cAMP - cGMP |
| Deoxynucleotides: dAMP - dTMP - dUMP - dGMP - dCMP - dADP - dTDP - dUDP - dGDP - dCDP - dATP - dTTP - dUTP - dGTP - dCTP |
| Nucleic acids: DNA - RNA - LNA - PNA - mRNA - ncRNA - miRNA - rRNA - siRNA - tRNA - mtDNA - Oligonucleotide |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.