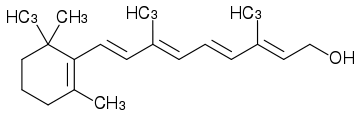
Vitamin A is a fat-soluble vitamin that belongs to a family of similarly shaped molecules, the retinoids, and occurs in several chemical forms, notably an aldehyde (retinal), an alcohol (retinol), and an acid (retinoic acid). In foods of animal origin, the major form of vitamin A is an ester, primarily retinyl palmitate, which is converted to retinol. Precursors to the vitamin (provitamins) are present in foods of plant origin as some of the members of the carotenoid family of compounds (Berdanier 1997).
Vitamin A is an essential human nutrient for normal metabolic functioning in both the embryo and the adult, including normal cell growth and development and vision. However, it is readily available from a diversity of both plant and animal matter. Nonetheless, vitamin deficiency is not uncommon in the developing world, affecting millions of children around the world and with hundreds of thousands of cases of blindness every year traced to this deficiency (NIH 2006).
Overview and structure
Vitamins, such as vitamin A, are organic nutrients that are obtained through the diet and are essential in small amounts for normal metabolic reactions. Vitamins can act both as catalysts and participants in chemical reactions.
Vitamin A actually refers to a family of similarly shaped molecules: The retinoids. The basic structure of the retinoid molecule consist of a cyclic end group, a polyene side chain and a polar end group. The conjugated system formed by alternating C=C double bonds in the polyene side chain are responsible for the color of retinoids (typically yellow, orange, or red). Hence, many retinoids are chromophores. Alternation of side chains and end groups creates the various classes of retinoids. The important part of vitamin A is the retinyl group, which can be found in several forms.
In foods of animal origin, the major form of vitamin A is an ester, primarily retinyl palmitate, which is converted to an alcohol (retinol) in the small intestine. Vitamin A can also exist as an aldehyde (retinal), or as an acid (retinoic acid).
In various plants, there are precursors to vitamin A in the form of some of the members of the carotenoid family of compounds. Carotenoids are organic pigments that are naturally occurring in chromoplasts of plants. Carotenoids belong to the category of tetraterpenoids (that is, they contain 40 carbon atoms). Structurally they are in the form of a polyene chain which is sometimes terminated by rings. Fewer than ten percent of the 563 identified carotenoids can be made into vitamin A in the body (NIH 2006).
Vitamin from an animal source is known as preformed vitamin A. Vitamin A found in fruits and vegetables, which can be made into retinol in the body, is known as provitamin A carotenoid (NIH 2006).
All forms of vitamin A have a Beta-ionone ring to which an isoprenoid chain is attached. This structure is essential for vitamin activity (Berdanier 1997). The orange pigment of carrot (Beta-carotene) can be represented as two connected retinyl groups. The retinyl group, when attached to a specific protein, is the only primary light absorber in visual perception, and the compound name is related to the retina of the eye.
The major source of retinoids from the diet are retinyl esters derived from animal sources. Retinyl esters are hydrolyzed in the intestinal lumen to yield free retinol and the corresponding fatty acid (that is, palmitate or stearate). After hydrolysis, retinol is taken up by the enterocytes. Retinyl ester hydrolysis requires the presence of bile salts that serve to solubilize the retinyl esters in mixed micelles and to activate the hydrolyzing enzymes (Stipanuk 2006).
Discovery of vitamin A
The discovery of vitamin A stemmed from research dating back to 1906, indicating that factors other than carbohydrates, proteins, and fats were necessary to keep cattle healthy (Wolf 2001). By 1917, one of these substances was independently discovered by Elmer McCollum at the University of Wisconsin-Madison, and Lafayette Mendel and Thomas Osborne at Yale University. Since "water-soluble factor B" (Vitamin B) had recently been discovered, the researchers chose the name "fat-soluble factor A" (vitamin A) (Wolf 2001). Vitamin A was first synthesized, in 1947, by two Dutch chemists, David Adriaan van Dorp and Jozef Ferdinand Arens.
Sources of Vitamin A
Vitamin A is found naturally in many foods. Among the best animal sources of vitamin A are eggs, liver, butter, milk, and such fish as tuna, sardines, and herring (Brody 2004). The best plant sources are dark-green, orange, and yellow vegetables and fruits, such as spinach, carrots, and oranges, while cereals are poor sources (Brody 2004).
The following are some foods and their vitamin A amounts:
- Liver (beef, pork, chicken, turkey, fish) (6500 μg 722 percent)
- Carrots (835 μg 93 percent)
- Broccoli leaves (800 μg 89 percent)
- sweet potatoes (709 μg 79 percent)
- kale (681 μg 76 percent)
- butter (684 μg 76 percent)
- spinach (469 μg 52 percent)
- leafy vegetables
- pumpkin (369 μg 41 percent)
- collard greens (333 μg 37 percent)
- cantaloupe melon (169 μg 19 percent)
- eggs (140 μg 16 percent)
- apricots (96 μg 11 percent)
- papaya (55 μg 6 percent)
- mango (38 μg 4 percent)
- peas (38 μg 4 percent)
- broccoli (31 μg 3 percent)
- winter squash
Note: bracketed values are retinol equivalences and percentage of the adult male RDA per 100g.
However, the figures for fruits and vegetables is somewhat misleading as absorption and conversion from plant sources is lower than once thought. Conversion of carotene to retinol varies from person to person and bioavailability of carotene in food varies (Borel et al. 2005; Tang et al. 2005).
Equivalencies of retinoids and carotenoids (IU)
Since some carotenoids from plant matter can be converted into vitamin A, attempts have been made to determine how much dietary carotenoid is equivalent to a particular amount of retinol, so that comparisons can be made of the benefit of different foods. Unfortunately the situation is confusing because the accepted equivalences have changed. For many years, a system of equivalencies was used in which an international unit (IU) was equal to 0.3 micrograms of retinol, 0.6 μg of β-carotene, or 1.2 μg of other provitamin-A carotenoids (ARS 2008). Later, a unit called retinol equivalent (RE) was introduced. One retinol equivalent correspond to 1 μg retinol, 2 μg β-carotene dissolved in oil (as in supplement pills), 6 μg β-carotene in normal food (because it is not absorbed as well as from supplements), and 12 μg of either α-carotene or β-cryptoxanthin in food.
However, new research showed that the absorption of provitamin-A carotenoids was only half as much as previously thought, so in 2001 the US Institute of Medicine recommended a new unit, the retinol activity equivalent (RAE). One μg RAE corresponds to 1 μg retinol, 2 μg of β-carotene in oil, 12 μg of "dietary" beta-carotene, or 24 μg of other dietary provitamin-A carotenoids (IM 2001).
| Substance and its chemical environment | Micrograms of retinol equivalent per microgram of the substance |
|---|---|
| retinol | 1 |
| beta-carotene, dissolved in oil | 1/2 |
| beta-carotene, common dietary | 1/12 |
| alpha-carotene, common dietary | 1/24 |
| beta-cryptoxanthin, common dietary | 1/24 |
Because the production of retinol from provitamins by the human body is regulated by the amount of retinol available to the body, the conversions apply strictly only for vitamin A deficient humans. The absorption of provitamins also depends greatly on the amount of lipids ingested with the provitamin; lipids increase the uptake of the provitamin (Solomons and Orozco 2003).
The conclusion that can be drawn from the newer research is that fruits and vegetables are not as useful for obtaining vitamin A as was thought—in other words, the IU's that they were reported to contain were worth much less than the same number of IU's of fat-dissolved supplements. This is important for vegetarians. (Night blindness is prevalent in countries where little meat or vitamin A-fortified foods are available.) A sample vegan diet for one day that provides sufficient vitamin A has been published by the Food and Nutrition Board (IM 2001). On the other hand, reference values for retinol or its equivalents, provided by the National Academy of Sciences, have decreased. The RDA (for men) of 1968 was 5000 IU (1500 μg retinol). In 1974, the RDA was set to 1000 RE (1000 μg retinol), whereas now the Dietary Reference Intake (DRI) is 900 RAE (900 μg or 3000 IU retinol). This is equivalent to 1800 μg of β-carotene supplement (3000 IU) or 10800 μg of β-carotene in food (18000 IU).
Recommended daily intake
Vitamin A
Dietary Reference Intake:
| Life Stage Group | RDA/AI*
ug/day |
UL
ug/day |
|---|---|---|
| Infants
0-6 months |
400* 500* |
600 600 |
| Children
1-3 years |
300 400 |
600 900 |
| Males
9-13 years |
600 900 900 |
1700 2800 3000 |
| Females
9-13 years |
600 700 700 |
1700 2800 3000 |
| Pregnancy
<19 years |
750 770 |
2800 3000 |
| Lactation
<19 years |
1200 1300 |
2800 3000 |
RDA = Recommended Dietary Allowances
AI* = Adequate Intakes
UL = Upper Limit
Note that the limit refers to synthetic and natural retinoid forms of vitamin A.
According to the Institute of Medicine of the National Academies, "RDAs are set to meet the needs of almost all (97 to 98 percent) individuals in a group. For healthy breastfed infants, the AI is the mean intake. The AI for other life stage and gender groups is believed to cover the needs of all individuals in the group, but lack of data prevent being able to specify with confidence the percentage of individuals covered by this intake" (IM 2001).
Metabolic functions of Vitamin A
Vitamin A plays a role in a variety of functions throughout the human body, such as:
- Vision
- Gene transcription
- Immune function
- Embryonic development and reproduction
- Bone metabolism
- Haematopoiesis
- Skin health
- Reducing risk of heart disease and cancer
- Antioxidant activity
Vitamin A is important for regulating the development of various tissues, such as the cells of the skin and lining of the respiratory, intestinal, and urinary tracts (Brody 2004; NIH 2006). If these linings break down or the skin and mucous membranes, then it because easier for bacteria and viruses to enter the body and cause infection (NIH 2006). In embryological development, a fertilized egg will not develop into a fetus without vitamin A (Brody 2004).
Vision
Vitamin A is an important component of the eye’s light-sensitive components that allow for night-vision and seeing in dim-light conditions (Brody 2004).
The role of vitamin A in the vision cycle is specifically related to the retinal form. Within the human eye, 11-cis-retinal is bound to rhodopsin (rods) and iodopsin (cones) at conserved lysine residues. As light enters the eye, the 11-cis-retinal is isomerized to the all-"trans" form. The all-"trans" retinal dissociates from the opsin in a series of steps called bleaching. This isomerization induces a nervous signal along the optic nerve to the visual center of the brain. Upon completion of this cycle, the all-"trans"-retinal can be recycled and converted back to the 11-"cis"-retinal form via a series of enzymatic reactions. Additionally, some of the all-"trans" retinal may be converted to all-"trans" retinol form and then transported with an interphotoreceptor retinol-binding protein (IRBP) to the pigment epithelial cells. Further esterification into all-"trans" retinyl esters allow this final form to be stored within the pigment epithelial cells to be reused when needed (Combs 2008). The final conversion of 11-cis-retinal will rebind to opsin to reform rhodopsin in the retina.
Rhodopsin is needed to see black and white as well as see at night. It is for this reason that a deficiency in vitamin A will inhibit the reformation of rhodopsin and lead to night blindness (McGuire and Beerman 2007).
Gene transcription
Vitamin A, in the retinoic acid form, plays an important role in gene transcription. Once retinol has been taken up by a cell, it can be oxidized to retinal (by retinol dehydrogenases) and then retinal can be oxidized to retinoic acid (by retinal oxidase). The conversion of retinal to retinoic acid is an irreversible step, meaning that the production of retinoic acid is tightly regulated, due to its activity as a ligand for nuclear receptors (Combs 2008).
Retinoic acid can bind to two different nuclear receptors to initiate (or inhibit) gene transcription: The retinoic acid receptors (RARs) or the retinoid "X" receptors (RXRs). RAR and RXR must dimerize before they can bind to the DNA. RAR will form a heterodimer with RXR (RAR-RXR), but it does not readily form a homodimer (RAR-RAR). RXR, on the other hand, readily forms a homodimer (RXR-RXR) and will form heterodimers with many other nuclear receptors as well, including the thyroid hormone receptor (RXR-TR), the Vitamin D3 receptor (RXR-VDR), the peroxisome proliferator-activated receptor (RXR-PPAR), and the liver "X" receptor (RXR-LXR) (Stipanuk 2006). The RAR-RXR heterodimer recognizes retinoid acid response elements (RAREs) on the DNA whereas the RXR-RXR homodimer recognizes retinoid "X" response elements (RXREs) on the DNA. The other RXR heterodimers will bind to various other response elements on the DNA (Combs 2008). Once the retinoic acid binds to the receptors and dimerization has occurred, the receptors undergo a conformational change that causes co-repressors to dissociate from the receptors. Coactivators can then bind to the receptor complex, which may help to loosen the chromatin structure from the histones or may interact with the transcriptional machinery (Stipanuk 2006). The receptors can then bind to the response elements on the DNA and upregulate (or downregulate) the expression of target genes, such as cellular retinol-binding protein (CRBP) as well as the genes that encode for the receptors themselves (Combs 2008).
Dermatology
Vitamin A appears to function in maintaining normal skin health. The mechanisms behind retinoid's therapeutic agents in the treatment of dermatological diseases are being researched. For the treatment of acne, the most effective drug is 13-cis retinoic acid (isotretinoin). Although its mechanism of action remains unknown, it is the only retinoid that dramatically reduces the size and secretion of the sebaceous glands. Isotretinoin reduces bacterial numbers in both the ducts and skin surface. This is thought to be a result of the reduction in sebum, a nutrient source for the bacteria. Isotretinoin reduces inflammation via inhibition of chemotatic responses of monocytes and neutrophils (Combs 2008). Isotretinoin also has been shown to initiate remodeling of the sebaceous glands; triggering changes in gene expression that selectively induces apoptosis (Nelson et al. 2008). Isotretinoin is a teratogen and its use is confined to medical supervision.
Vitamin A deficiency
Vitamin A deficiency is estimated to affect millions of children around the world. Approximately 250,000 to 500,000 children in developing countries become blind each year owing to vitamin A deficiency, with the highest prevalence in Southeast Asia and Africa (NIH 2006). According to the World Health Organization (WHO), vitamin A deficiency is under control in the United States, but in developing countries vitamin A deficiency is a significant concern. With the high prevalence of vitamin A deficiency, the WHO has implemented several initiatives for supplementation of vitamin A in developing countries. Some of these strategies include intake of vitamin A through a combination of breast feeding, dietary intake, food fortification, and supplementation. Through the efforts of WHO and its partners, an estimated 1.25 million deaths since 1998 in 40 countries due to vitamin A deficiency have been averted (WHO 2008).
Vitamin A deficiency can occur as either a primary or secondary deficiency. A primary vitamin A deficiency occurs among children and adults who do not consume an adequate intake of yellow and green vegetables, fruits, liver, and other sources of vitamin A. Early weaning can also increase the risk of vitamin A deficiency.
Secondary vitamin A deficiency is associated with chronic malabsorption of lipids, impaired bile production and release, low fat diets, and chronic exposure to oxidants, such as cigarette smoke. Vitamin A is a fat soluble vitamin and depends on micellar solubilization for dispersion into the small intestine, which results in poor utilization of vitamin A from low-fat diets. Zinc deficiency can also impair absorption, transport, and metabolism of vitamin A because it is essential for the synthesis of the vitamin A transport proteins and the oxidation of retinol to retinal. In malnourished populations, common low intakes of vitamin A and zinc increase the risk of vitamin A deficiency and lead to several physiological events (Combs 2008). A study in Burkina Faso showed major reduction of malaria morbidity with combined vitamin A and zinc supplementation in young children (Zeba et al. 2008).
Since the unique function of retinyl group is the light absorption in retinylidene protein, one of the earliest and specific manifestations of vitamin A deficiency is impaired vision, particularly in reduced light—Night blindness. Persistent deficiency gives rise to a series of changes, the most devastating of which occur in the eyes. Some other ocular changes are referred to as xerophthalmia. First there is dryness of the conjunctiva (xerosis) as the normal lacrimal and mucus secreting epithelium is replaced by a keratinized epithelium. This is followed by the build-up of keratin debris in small opaque plaques (Bitot's spots) and, eventually, erosion of the roughened corneal surface with softening and destruction of the cornea (keratomalacia) and total blindness (Roncone 2006).Other changes include impaired immunity, hypokeratosis (white lumps at hair follicles), keratosis pilaris, and squamous metaplasia of the epithelium lining the upper respiratory passages and urinary bladder to a keratinized epithelium. With relations to dentistry, a deficiency in Vitamin A leads to enamel hypoplasia.
Adequate supply of Vitamin A is especially important for pregnant and breastfeeding women, since deficiencies cannot be compensated by postnatal supplementation (Strobel et al. 2007; Schulz et al. 2007).
Toxicity
As vitamin A is fat-soluble, disposing of any excesses taken in through diet is much harder than with water-soluble vitamins B and C. As such, vitamin A toxicity can result. This can lead to nausea, jaundice, irritability, anorexia (not to be confused with anorexia nervosa, the eating disorder), vomiting, blurry vision, headaches, muscle and abdominal pain, and weakness, drowsiness, and altered mental status.
Acute toxicity generally occurs at doses of 25,000 IU/kilogram of body weight, with chronic toxicity occurring at 4,000 IU/kilogram of body weight daily for 6-15 months (Rosenbloom 2007). However, liver toxicities can occur at levels as low as 15,000 IU per day to 1.4 million IU per day, with an average daily toxic dose of 120,000 IU per day. In people with renal failure 4000 IU can cause substantial damage. Additionally excessive alcohol intake can increase toxicity. Children can reach toxic levels at 1500IU/kg of body weight (Penniston and Tanumihardjo 2006).
In chronic cases, hair loss, drying of the mucous membranes, fever, insomnia, fatigue, weight loss, bone fractures, anemia, and diarrhea can all be evident on top of the symptoms associated with less serious toxicity (Eledrisi 2008). Chronically high doses of Vitamin A can produce the syndrome of "pseudotumor cerebri." This syndrome includes headache, blurring of vision and confusion. It is associated with increased intracerebral pressure (Giannini and Gilliland 1982).
It has been estimated that 75 percent of people may be ingesting more than the RDA for vitamin A on a regular basis in developed nations. Intake of twice the RDA of preformed vitamin A chronically may be associated with osteoporosis and hip fractures. High vitamin A intake has been associated with spontaneous bone fractures in animals. Cell culture studies have linked increased bone resorption and decreased bone formation with high vitamin A intakes. This interaction may occur because vitamins A and D may compete for the same receptor and then interact with parathyoid hormone which regulates calcium (Penniston and Tanumihardjo 2006).
Toxic effects of vitamin A have been shown to significantly affect developing fetuses. Therapeutic doses used for acne treatment have been shown to disrupt cephalic neural cell activity. The fetus is particularly sensitive to vitamin A toxicity during the period of organogenesis (Combs 2008).
These toxicities only occur with preformed (retinoid) vitamin A (such as from liver). The carotenoid forms (such as beta-carotene as found in carrots), give no such symptoms, but excessive dietary intake of beta-carotene can lead to carotenodermia, which causes orange-yellow discoloration of the skin (Sale and Stratman 2004; Nishimura et al. 1998; Takita et al. 2006).
A correlation also has been shown between low bone mineral density and too high intake of vitamin A (Forsmo et al. 2008).
Researchers have succeeded in creating water-soluble forms of vitamin A, which they believed could reduce the potential for toxicity (Wicklegren 1989). However, a 2003 study found that water-soluble vitamin A was approximately 10 times as toxic as fat-soluble vitamin (Myhre et al. 2003). A 2006 study found that children given water-soluble vitamin A and D, which are typically fat-soluble, suffer from asthma twice as much as a control group supplemented with the fat-soluble vitamins (Kull et al. 2006).
ReferencesISBN links support NWE through referral fees
- Agricultural Research Service (ARS). 2008. Composition of foods raw, processed, prepared. USDA National Nutrient Database for Standard Reference, Release 20. Agricultural Research Service, U.S. Department of Agriculture. Retrieved September 7, 2008.
- Berdanier, C. 1997. Advanced Nutrition Micronutrients. Boca Raton, Fla: CRC Press. ISBN 0849326648.
- Borel, P., J. Drai, H. Faure, et al. 2005. Recent knowledge about intestinal absorption and cleavage of carotenoids. Ann. Biol. Clin 63(2):165–77. PMID 15771974. Retrieved September 7, 2008.
- Brody, T. 2004. Vitamin A deficiency. Pages 3512-3513 in J. L. Longe, The Gale Encyclopedia of Medicine, 2nd ed. Detroit: Gale Group/Thomson Learning. ISBN 0787654949.
- Combs, G. F. 2008. The Vitamins: Fundamental Aspects in Nutrition and Health, 3rd ed. Burlington: Elsevier Academic Press. ISBN 9780121834937.
- Eledrisi, M. S. 2008. Vitamin A toxicity. eMedicine. Retrieved September 7, 2008.
- Forsmo, S., S. K. Fjeldbo, and A. Langhammer. 2008. Childhood cod liver oil consumption and bone mineral density in a population-based cohort of peri- and postmenopausal women: The Nord-Trøndelag Health Study. American Journal of Epidemiology 167(4): 406-411. PMID 18033763. Retrieved September 7, 2008.
- Giannini, A. J., and R. L. Gilliland. 1982. The Neurologic, Neurogenic and Neuropsychiatric Disorders Handbook. New Hyde Park, NY. Medical Examination Publishing. ISBN 0874886996.
- Institute of Medicine, United States (IM). 2001. Chapter 4: Vitamin A. In Dietary Reference Intakes (DRI) for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc]: A Report of the Panel on Micronutrients ... and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Washington, D.C.: National Academy Press. ISBN 0309072794.
- Kull, I., A. Bergström, E. Melén, et al. 2006. Early-life supplementation of vitamins A and D, in water-soluble form or in peanut oil, and allergic diseases during childhood. J. Allergy Clin. Immunol. 118(6): 1299–304. PMID 17157660. Retrieved September 6, 2008.
- McGuire, M., and K. A. Beerman. 2007. Nutritional Sciences: From Fundamentals to Food. Belmont, CA: Thomson/Wadsworth. ISBN 0534537170.
- Myhre, A. M., M. H. Carlsen, S. K. Bøhn, H. L. Wold, P. Laake, and R. Blomhoff. 2003. Water-miscible, emulsified, and solid forms of retinol supplements are more toxic than oil-based preparations. Am. J. Clin. Nutr. 78(6): 1152–9. PMID 14668278. Retrieved September 7, 2008.
- National Institute of Health (NIH), Office of Dietary Supplements (ODS). 2006. Dietary supplement fact sheet: Vitamin A and carotenoids. National Institute of Health. Retrieved September 7, 2008.
- Nelson, A. M., W. Zhao, K. L. Gilliland, et al. 2008. Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells. Journal of Clinical Investigation 118(4): 1468-1478. Retrieved September 7, 2008.
- Nishimura, Y., N. Ishii, Y. Sugita, and H. Nakajima. 1998. A case of carotenodermia caused by a diet of the dried seaweed called Nori. J. Dermatol. 25(10): 685–7. PMID 9830271.
- Penniston, K. L., and S. A. Tanumihardjo. 2006. The acute and chronic toxic effects of vitamin A. American Journal of Clinical Nutrition 83(2): 191–201. PMID 16469975. Retrieved September 7, 2008.
- Roncone, D. P. 2006. Xerophthalmia secondary to alcohol-induced malnutrition. Optometry 77(3): 124–33. PMID 16513513. Retrieved September 7, 2008.
- Rosenbloom, M. 2007. Toxicity, vitamin. eMedicine. Retrieved September 7, 2008.
- Sale, T. A., and E. Stratman. 2004. Carotenemia associated with green bean ingestion. Pediatr Dermatol 21(6): 657–9. PMID 15575851. Retrieved September 7, 2008.
- Schulz, C., U. Engel, R. Kreienberg, and H. K. Biesalski. 2007. Vitamin A and beta-carotene supply of women with gemini or short birth intervals: A pilot study. Eur J Nutr 46(1): 12–20. PMID 17103079. Retrieved September 7, 2008.
- Solomons, N. W., and M. Orozco. 2003. Alleviation of vitamin A deficiency with palm fruit and its products. Asia Pac J Clin Nutr 12(3): 373-84.
- Stipanuk, M. H. 2006. Vitamin A: Biochemical, Physiological, and Molecular Aspects of Human Nutrition. Philadelphia, PA: Elsevier Saunders. ISBN 141600209X.
- Strobel, M., J. Tinz, and H. K. Biesalski. 2007. The importance of beta-carotene as a source of vitamin A with special regard to pregnant and breastfeeding women. Eur J Nutr 46(Suppl 1): I1–20. PMID 17665093. Retrieved September 7, 2008.
- Takita, Y., M. Ichimiya, Y. Hamamoto, and M. Muto. 2006. A case of carotenemia associated with ingestion of nutrient supplements. J. Dermatol. 33(2): 132–4. PMID 16556283. Retrieved September 7, 2008.
- Tang, G., J. Qin, G. G. Dolnikowski, R. M. Russell, and M. A. Grusak. 2005. Spinach or carrots can supply significant amounts of vitamin A as assessed by feeding with intrinsically deuterated vegetables. Am. J. Clin. Nutr. 82(4): 821–8. PMID 16210712. Retrieved September 7, 2008.
- Wicklegren, I. 1989. http://findarticles.com/p/articles/mi_m1200/is_n13_v135/ai_7502207 Water-soluble vitamin A shows promise.] Science News April 1, 1989. Retrieved September 7, 2008.
- Wolf, G. 2001. Discovery of vitamin A. Encyclopedia of Life Sciences. Hoboken, NJ : John Wiley & Sons. Retrieved September 7, 2008.
- World Health Organization (WHO). 2008. Micronutrient deficiencies: Vitamin A. World Health Organization. Retrieved September 7, 2008.
- Zeba, A. N., h. Sorgho, N. Rouamba, et al. 2008. Major reduction of malaria morbidity with combined vitamin A and zinc supplementation in young children in Burkina Faso: A randomized double blind trial. Nutr J 7: 7. PMID 18237394. Retrieved September 7, 2008.
| Vitamins |
|---|
| All B vitamins | All D vitamins |
| Retinol (A) | Thiamine (B1) | Riboflavin (B2) | Niacin (B3) | Pantothenic acid (B5) | Pyridoxine (B6) | Biotin (B7) | Folic acid (B9) | Cyanocobalamin (B12) | Ascorbic acid (C) | Ergocalciferol (D2) | Cholecalciferol (D3) | Tocopherol (E) | Naphthoquinone (K) |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.