Xenon
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
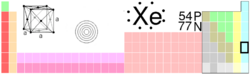
| Name, Symbol, Number | xenon, Xe, 54 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | noble gases | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 18, 5, p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | colorless 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 131.293(6) g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d10 5s2 5p6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 18, 8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | gas | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density | (0 °C, 101.325 kPa) 5.894 g/L | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 161.4 K (-111.7 °C, -169.1 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 165.03 K (-108.12 °C, -162.62 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Critical point | 289.77 K, 5.841 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 2.27 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 12.64 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat capacity | (25 °C) 20.786 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | cubic face centered | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 0, +1, +2, +4, +6, +8 (rarely more than 0) (weakly acidic oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.6 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 1170.4 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 2046.4 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 3099.4 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 108 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 130 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 216 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | nonmagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 5.65 mW/(m·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | (liquid) 1090 m/s | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-63-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Notable isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Xenon (chemical symbol Xe, atomic number 54) is a colorless, odorless, heavy noble gas that occurs in the Earth's atmosphere in trace amounts. It was the first noble gas from which a compound was synthesized successfully, and many xenon compounds have been made by now.[1] [2] Xenon flash lamps are widely used in flash photography, and xenon arc lamps are used in solar simulators and automotive high-intensity discharge (HID) headlights. In addition, xenon is the preferred fuel for ion propulsion, and it is used in bubble chambers in nuclear power plants. It is commonly used to analyze protein structures by crystallography, and some of its salts (called perxenates) are used as oxidizing agents in analytical chemistry.
Occurrence and extraction
Xenon is a trace gas in the Earth's atmosphere, occurring in one part in twenty million. In addition, it is found in gases emitted from some mineral springs.
This element can be extracted by fractional distillation of liquid air or by selective adsorption (surface binding) on activated carbon. The isotopes Xe-133 and Xe-135 are synthesized by neutron irradiation within air-cooled nuclear reactors.
History
Xenon (from the Greek word ξένος, meaning "strange") was discovered in England by William Ramsay and Morris Travers on July 12, 1898, shortly after they had discovered the elements krypton and neon. They found it in the residue left over from evaporating components of liquid air.
Notable characteristics
Xenon is a member of the noble gas series in the periodic table. It is situated between krypton and radon in group 18 (former group 8A), and is placed after iodine in period 5.
As the noble gases are chemically very inert, they are said to have a chemical valence of zero. Nonetheless, the term "inert" is not an entirely accurate description of this group of elements, because some of them—including xenon—have been shown to form compounds (see Compounds below).
In a gas-filled tube, xenon emits a blue glow when the gas is excited by electrical discharge. Using tens of gigapascals of pressure, xenon has been forced into a metallic phase.[3] Xenon can also form "clathrates" (cage-like molecules) with water, when xenon atoms are trapped in a lattice of water molecules.
Isotopes
Naturally occurring xenon is made of seven stable and two slightly radioactive isotopes. Twenty additional unstable isotopes have been studied. Xe-129 is produced by the beta decay of iodine-129 (half-life 16 million years); Xe-131m, Xe-133, Xe-133m, and Xe-135 are some of the nuclear fission products of both uranium-235 and plutonium-239, and therefore used as indicators of nuclear explosions.
The artificial isotope Xe-135 is of considerable significance in the operation of nuclear fission reactors. Xe-135 acts as a neutron absorber (or "poison") that can slow or stop the chain reaction after a period of operation. This was discovered in the earliest nuclear reactors built by the American Manhattan Project for plutonium production, but the designers had made provisions to circumvent this problem.
Relatively high concentrations of radioactive xenon isotopes have been found to emanate from nuclear reactors, because this fission gas is released from cracked fuel rods or fissioning of uranium in cooling water. The concentrations of these isotopes are still usually low compared to naturally occurring radioactive noble gases such as radon-222.
Given that xenon is a tracer for two parent isotopes, xenon isotope ratios in meteorites are a powerful tool for studying the formation of the Solar System. The I-Xe method of dating gives the time elapsed between nucleosynthesis and the condensation of a solid object from the solar nebula. Xenon isotopes are also a powerful tool for understanding the formation of the Earth. Excess Xe-129 found in carbon dioxide well gases from New Mexico was believed to be from the decay of mantle-derived gases soon after the Earth's formation.[4]
Compounds
Xenon and the other noble gases had long been considered completely chemically inert and unable to form compounds. In 1962, however, at the University of British Columbia, the first xenon compound—xenon hexafluoroplatinate—was synthesized successfully. Many compounds of xenon have been prepared by now, including xenon difluoride, xenon tetrafluoride, xenon hexafluoride, xenon tetroxide, xenon hydrate, xenon deuterate, and sodium perxenate. A highly explosive compound, xenon trioxide, has also been made. There are at least 80 xenon compounds in which fluorine or oxygen is bonded to xenon. Some xenon compounds are colored, but most are colorless.
Recently, researchers (M. Räsänen at al.) at the University of Helsinki in Finland made xenon dihydride (HXeH), xenon hydride-hydroxide (HXeOH), and hydroxenoacetylene (HXeCCH). These compounds are stable up to 40K.[5]
Applications
- Xenon is most widely used in light-emitting devices called xenon flash lamps (for flash photography), stroboscopic lamps, to excite the active medium in lasers, in bactericidal lamps (occasionally), and in certain dermatological uses.
- Certain xenon arc lamps are used in solar simulators, some projection systems, automotive high-intensity discharge (HID) lamp headlights, and other specialized devices. They are an excellent source of short-wavelength ultraviolet light, and they have intense emissions in the near infrared, which are used in some night vision systems.
- Xenon has been used as a general anesthetic, but the cost is extremely high.
- In nuclear energy applications, it is used in bubble chambers, probes, and in other areas where a high-molecular-weight, inert substance is needed.
- Xenon salts called perxenates are used as oxidizing agents in analytical chemistry.
- The isotope 129Xe is used for hyperpolarized MRI of the lungs and other tissues.[6]
- It is the preferred fuel for ion propulsion, because of its high molecular weight, ease of ionization, storability as a liquid near room temperature (but at high pressure), and easy convertibility back into a gas to fuel the engine. Its inert nature makes it environmentally friendly and less corrosive to an ion engine than other fuels such as mercury or cesium. Europe's SMART-1 spacecraft utilized xenon in its engines.[7]
- It is commonly used to analyze protein structures by crystallography. Xenon atoms can be bound to protein molecules in a crystal, creating a high quality, heavy-atom derivative that is then analyzed.
Precautions
Xenon gas can be safely stored in normal sealed glass containers at standard temperature and pressure. Xenon is nontoxic, but many of its compounds are toxic on account of their strong oxidative properties.
As xenon is denser than air, the speed of sound in xenon is slower than that in air. When inhaled, it lowers the resonant frequencies of the vocal tract, producing a characteristic lowered voice pitch (this is the opposite of the high-pitched voice caused by inhalation of helium.) Like helium, xenon does not satisfy the body's need for oxygen and is a simple asphyxiant. Consequently, many universities no longer allow the voice stunt as a general chemistry demonstration. As xenon is expensive, the gas sulfur hexafluoride, which is similar to xenon in molecular weight (146 vs. 131), is generally used in this stunt, although it too is an asphyxiant.
There is a myth that xenon is too heavy for the lungs to expel unassisted, and that after inhaling xenon, it is necessary to bend over completely at the waist to allow the excess gas to "spill" out of the body. In fact, the lungs mix gases very effectively and rapidly, such that xenon would be purged from the lungs within a breath or two. There is, however, a danger associated with any heavy gas in large quantities: it may sit invisibly in an unventilated space, and a person who enters the space may breathe it unknowingly. Xenon is rarely used in large enough quantities for this to be a concern, but the potential for danger exists any time a tank or container of xenon is kept in an unventilated space.
ReferencesISBN links support NWE through referral fees
- ↑ Los Alamos National Laboratory – Xenon
- ↑ Thermophysical properties of neon, argon, krypton, and xenon / V. A. Rabinovich ... Theodore B. Selover, English-language edition. Washington Hemisphere Publ. Corp., 1988.
- ↑ Nguyen, J., B. Pfrommer, S. Louie, and R. Jeanloz, “Structure, bonding and geochemistry of xenon at high pressures.” Science 277 (1997): 930-933.
- ↑ Boulos, M. S. “The xenon record of extinct radioactivities in the Earth.” Science 174 (1971): 1334-1336.
- ↑ See [1] in its paragraph starting "Many recent findings".
- ↑ Use of Xe in MRI
- ↑ CNN article on SMART-1 and xenon
External links
All links retrieved May 20, 2023.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.